Volume 14, Issue 4 (December 2016)
Iranian Rehabilitation Journal 2016, 14(4): 239-245 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghaemi H, Sobhani-Rad D, Arabi A, Saifpanahi S, Ghayoumi Anaraki Z. Role of Basal Ganglia in Swallowing Process: A Systematic Review. Iranian Rehabilitation Journal 2016; 14 (4) :239-245
URL: http://irj.uswr.ac.ir/article-1-637-en.html
URL: http://irj.uswr.ac.ir/article-1-637-en.html
1- Department of Speech Therapy, School of Rehabilitation, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Speech Therapy, School of Paramedical Sciences, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Speech Therapy, School of Rehabilitation Sciences, Hamedan University of Medical Sciences, Hamedan, Iran.
2- Department of Speech Therapy, School of Paramedical Sciences, Mashhad University of Medical Sciences, Mashhad, Iran.
3- Department of Speech Therapy, School of Rehabilitation Sciences, Hamedan University of Medical Sciences, Hamedan, Iran.
Full-Text [PDF 549 kb]
(6760 Downloads)
| Abstract (HTML) (6452 Views)
Full-Text: (5005 Views)
1. Introduction
Swallowing is defined as a complex sensorimotor behavior that requires the co-ordinated function of muscles located around the mouth, tongue, larynx, pharynx, and esophagus in order to transport food from the oral cavity to the stomach [1]. The volitional and automatic movements during swallowing, which are controlled by more than 30 nerves and muscles [2-4], could be divided into three inter-related physiological stages: [1] the oral stage that is voluntary and highly variable in duration; [2] the pharyngeal stage during which translocation of bolus occurs from the oropharynx (throat) into the esophagus without aspiration; and [3] esophageal stage in which the bolus moves through the lower esophageal sphincter into the stomach under the control of autonomic nervous system [5-10]. Accurate control of the swallowing function relies on the exact innervations of several areas in the brain, including the neo-cortex, sub-cortical regions, brainstem, and peripheral nervous system [11]. The brainstem swallowing center is known as the first level of swallowing control while the subcortical structures, such as basal ganglia (BG), hypothalamus, amygdala, and tegmental area of the midbrain, are in the next levels, and finally, there are supra-bulbar cortical swallowing centers [12].
BG is a group of interconnected nuclei including striatum (further subdivided into putamen and caudate nuclei), Sub-Thalamic Nucleus (STN), globus pallidus external and internal segments (GPe and GPi, respectively), and substantia nigra pars compacta and pars reticulate (SNc and SNr, respectively). BG plays a vital role in a variety of motor, cognitive, and limbic functions by integrating the information derived from multiple cortical regions and conveying it back to frontal cortical regions and brainstem nuclei after processing it [13,14].
Although varied investigations have clarified the various functions of the BG, its exact role in the swallowing process has not yet been fully comprehended. For instance, a number of reports have indicated that damages and lesions of BG might lead to some degree of swallowing disorders; but, little has been discussed about the probable mechanisms and pathways in this aspect. Hence, we decided to conduct a systematic review based on a number of clinical studies for a better understanding of BG’s function in swallowing.
2. Methods
Numerous English-language articles that were published up to December 2015 and included keywords like swallowing, basal ganglia, swallowing neurology, neuro-imaging, dysphasia, and neurogenic dysphasia in their title or abstract were extracted from databases such as PubMed, Willy, Springer, and Elsevier (Medline, EBMR, Google Scholar, Science Direct, and ProQuest). Among these articles, eligible studies related to “neurological aspects of swallowing” and/or “lesions of subcortical or BG relevant to swallowing disorders” were filtered out in accordance with our pre-decided inclusion criteria. Conversely, papers with no focus on BG and swallowing problems were excluded.
3. Results
The current systemic review revealed that despite extensive data published on swallowing neurology, only a few studies have focused on the topic considered here. For instance, the role of BG in the process of swallowing has been examined in papers on hemorrhagic BG [15], stroke [16-18], dementia, and traumatic brain injury [19, 20]. Some studies have also focused on the role of the extra-pyramidal syndrome, such as Parkinson’s, Huntington’s, and Wilson’s diseases in the swallowing process [21-24]. In addition, there are a few reports on the swallowing performance in elderly individuals [25-28].
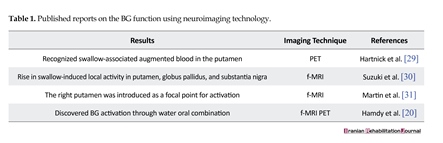
These limited publications indicate that swallowing is a sophisticated process with several unknown aspects. Nevertheless, it is necessary to mention that developments in neuroimaging techniques have improved our knowledge about BG functionality throughout the swallowing process, which has been detected through cryptogram of the BG function in brain images during swallowing. As summarized in Table 1, neuroimaging studies have confirmed the fact that swallowing involves the activation of multiple areas in the human brain, including putamen, globus pallidus, substantia nigra, and BG.
Based on the reviewed papers, BG is linked to sensorimotor, supplementary motor, pre-motor, associative and limbic cortices through functionally related loops [32-35], and such somatotopic organization supports both motor and cognitive functions [36]. BG receives input from sensorimotor areas of the cerebral cortex, which are primary and secondary somato-sensory, primary motor cortex, and premotor areas [37, 38]. In addition, BG has extensive connections with the thalamus and other sub-cortical structures [39, 40].
Swallowing is defined as a complex sensorimotor behavior that requires the co-ordinated function of muscles located around the mouth, tongue, larynx, pharynx, and esophagus in order to transport food from the oral cavity to the stomach [1]. The volitional and automatic movements during swallowing, which are controlled by more than 30 nerves and muscles [2-4], could be divided into three inter-related physiological stages: [1] the oral stage that is voluntary and highly variable in duration; [2] the pharyngeal stage during which translocation of bolus occurs from the oropharynx (throat) into the esophagus without aspiration; and [3] esophageal stage in which the bolus moves through the lower esophageal sphincter into the stomach under the control of autonomic nervous system [5-10]. Accurate control of the swallowing function relies on the exact innervations of several areas in the brain, including the neo-cortex, sub-cortical regions, brainstem, and peripheral nervous system [11]. The brainstem swallowing center is known as the first level of swallowing control while the subcortical structures, such as basal ganglia (BG), hypothalamus, amygdala, and tegmental area of the midbrain, are in the next levels, and finally, there are supra-bulbar cortical swallowing centers [12].
BG is a group of interconnected nuclei including striatum (further subdivided into putamen and caudate nuclei), Sub-Thalamic Nucleus (STN), globus pallidus external and internal segments (GPe and GPi, respectively), and substantia nigra pars compacta and pars reticulate (SNc and SNr, respectively). BG plays a vital role in a variety of motor, cognitive, and limbic functions by integrating the information derived from multiple cortical regions and conveying it back to frontal cortical regions and brainstem nuclei after processing it [13,14].
Although varied investigations have clarified the various functions of the BG, its exact role in the swallowing process has not yet been fully comprehended. For instance, a number of reports have indicated that damages and lesions of BG might lead to some degree of swallowing disorders; but, little has been discussed about the probable mechanisms and pathways in this aspect. Hence, we decided to conduct a systematic review based on a number of clinical studies for a better understanding of BG’s function in swallowing.
2. Methods
Numerous English-language articles that were published up to December 2015 and included keywords like swallowing, basal ganglia, swallowing neurology, neuro-imaging, dysphasia, and neurogenic dysphasia in their title or abstract were extracted from databases such as PubMed, Willy, Springer, and Elsevier (Medline, EBMR, Google Scholar, Science Direct, and ProQuest). Among these articles, eligible studies related to “neurological aspects of swallowing” and/or “lesions of subcortical or BG relevant to swallowing disorders” were filtered out in accordance with our pre-decided inclusion criteria. Conversely, papers with no focus on BG and swallowing problems were excluded.
3. Results
The current systemic review revealed that despite extensive data published on swallowing neurology, only a few studies have focused on the topic considered here. For instance, the role of BG in the process of swallowing has been examined in papers on hemorrhagic BG [15], stroke [16-18], dementia, and traumatic brain injury [19, 20]. Some studies have also focused on the role of the extra-pyramidal syndrome, such as Parkinson’s, Huntington’s, and Wilson’s diseases in the swallowing process [21-24]. In addition, there are a few reports on the swallowing performance in elderly individuals [25-28].
These limited publications indicate that swallowing is a sophisticated process with several unknown aspects. Nevertheless, it is necessary to mention that developments in neuroimaging techniques have improved our knowledge about BG functionality throughout the swallowing process, which has been detected through cryptogram of the BG function in brain images during swallowing. As summarized in Table 1, neuroimaging studies have confirmed the fact that swallowing involves the activation of multiple areas in the human brain, including putamen, globus pallidus, substantia nigra, and BG.
Based on the reviewed papers, BG is linked to sensorimotor, supplementary motor, pre-motor, associative and limbic cortices through functionally related loops [32-35], and such somatotopic organization supports both motor and cognitive functions [36]. BG receives input from sensorimotor areas of the cerebral cortex, which are primary and secondary somato-sensory, primary motor cortex, and premotor areas [37, 38]. In addition, BG has extensive connections with the thalamus and other sub-cortical structures [39, 40].
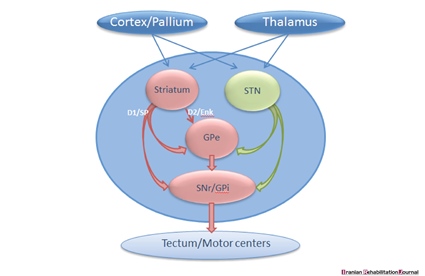
As presented in Figure 1, BG (striatum) receives input from all cortical areas and projects through the thalamus to prefrontal, premotor, and supplementary motor areas that are involved in motor planning. In the BG-thalamo-cortical circuits, the thalamus acts as a sensory-relay station and conveys information about the sensation of eating and swallowing to other cortical and sub-cortical structures [39]. Both voluntary and involuntary movements happening during ingestion are further modified by the feedbacks received from kinesthetic images and other afferents converging onto the BG, which further monitor and refine the movement progression in order to ensure the temporal and spatial accuracy [41]. Finally, BG notifies about the movement-related cortices involved in the preparation for next act while promoting muscle relaxation [42].
4. Discussion
Swallowing is a multi-faceted performance with active neural synchronization at the cerebral and brainstem levels. Studies on functional magnetic resonance imaging (fMRI) have recognized the anatomic sections, which are active during swallowing, including the chief sensory and motor cortex, additional motor area (SMA), cingulate cortex, insula, operculum, prefrontal and inferior frontal cortices, BG, thalamus, and cerebellum [43-48]. As mentioned earlier, automatic movements of swallowing are controlled by BG that establishes accurate timing and spacing in this process [37, 38].
Although the rhythmic pattern of mastication is controlled by the central pattern generator in the brainstem, it is supplemented by the motor cortex that provides pre-programmed movement patterns based on expectations and sensory feedbacks in conjunction with BG. The production level of BG includes active GABAergic neurons arising from two nuclei, GPi and SNr. The sub-populations of GABAergic neurons from the structure have outstanding projections to diverse motor cores in the brainstem [49]. As these neurons are tonically energetic at rest, they can sustain the incessant inhibitory drive [50-56].
Therefore, starting a motor program similar to swallowing depends on the elimination of such tonic reticence; hence, the pallidal output neurons must be inhibited from the input layer of BG [51, 53, 57-59]. Beyond the pallidal control of motor centers, the neurons projected by pallidum returns to the groups of cells inside the thalamus and are further projected back to the cortex. The pallido-thalamo-cortical loop controls emotions, swallowing, and motor and cognitive functions [60]. It is worth mentioning that in neurological diseases like Parkinson’s, the cortex is out of the loop, and hence, all the actions of BG are done straightforwardly over brainstem targets [61].
It is also essential to review the BG-nuclei that manage the output level as discussed in previous studies. As demonstrated in Figure 2, the projection neurons in the striatum are divided into two groups. Firstly are the dopamine receptors D1 type (D1R) that project straightforwardly to subpopulations of neurons at the production level (SNr/GPi) and get involved in the beginning of motor programs of swallowing, and secondly are the dopamine D2 receptors (D2R) that project to GPe - a neural structure that contributes with STN and restrain movements [61-63].
The subpopulation of D1R projected neurons control the fundamental aspects of motor performance of swallowing and depend on the excitatory inputs from thalamus and cortex/pallium, which further verifies whether they are activated or not. In case activated, the D1R neurons will participate in the instigation of a given motor program for swallowing [64, 65]. The tonic level of dopamine discharge resolves the responsiveness of the striatal neurons, and thus, the negligible dopamine makes it intricate to activate the movements, similar to that observed in Parkinson’s disease [66]. Dopamine neurons of BG have another characteristic importance in the motor system, i.e., they react with short-lasting bursts of activity throughout the awareness - a trait that can be important in promoting the knowledge of swallowing behavior [67, 68]. Despite their significance, our understanding of neural circuits, which are responsible for the value-based changes in dopamine discharge, is not complete [69-71].
It is, therefore, important to mention the dissimilar components inside the BG-controlled special motor programs. The swallowing process depends on the input from pallium/cortex, thalamus and the dopamine system and includes various parts of both direct (D1) and indirect pathways (D2). The production cells from GPi and SNr aim at the diverse motor centers [55, 71]. The selection of specific units depends on the excitatory input from thalamus and pallium/cortex along with the degree of tonic dopamine activity, which collectively define the prototype of BG’s behavior. In addition varied other motor patterns can be shared, for instance, one can swallow and chew simultaneously while one can only turn left or right and not both at the same time [59].
Therefore, mechanisms must be discovered for governing different behaviors of BG. Nevertheless, it is obvious that the BG plays a key role in making a flat series of movements [65]. Therefore, movement skills are compromised in patients with Parkinson’s disease, and there is a propensity to carry out merely one motor pattern at a time [72].
In this review study, we aimed at clarifying the role of the BG in the swallowing process for the first time. Reviewing of the published data on neurological control in swallowing process revealed that BG is one of the most complicated neurological structures, partially due to its location in the brain with indistinct performances. On the other hand, few published studies have focused on neurological aspects of swallowing, indicating that it is a sophisticated process with several unknown aspects. However, with the help of neuroimaging techniques, it has been confirmed that BG is linked to neural structures that support motor and cognitive functions such as the one involved in swallowing. BG receives input from all the cortical areas and projects to prefrontal, pre-motor, and supplementary motor areas through the thalamus. In BG-thalamo-cortical circuits, the thalamus conveys the information about the sensation of eating and swallowing to other structures while BG monitors the movement of progression to ensure the accuracy of swallowing from its different aspects.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to thank Mr. Mohsen Ghiasi for designing the graphics of the article.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clinical Neurophysiology. 2003; 114(12):2226-44. doi: 10.1016/s1388-2457(03)00237-2
Ertekin C. Physiological and pathological aspects of oropharyngeal swallowing. Movement Disorders. 2002; 17(S2):S86–S89. doi: 10.1002/mds.10068
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: Normal and abnormal. Physical Medicine and Rehabilitation Clinics of North America. 2008; 19(4):691–707. doi: 10.1016/j.pmr.2008.06.001
Jones B. Normal and abnormal swallowing: Imaging in diagnosis and therapy. Philadelphia: Springer; 2003.
Bakheit AMO. Management of neurogenic dysphagia. Postgraduate Medical Journal. 2001; 7(913):694–9. doi: 10.1136/pmj.77.913.694
Ertekin C, Palmer JB. Chapter 19 Physiology and electromyography of swallowing and its disorders. Supplements to Clinical Neurophysiology. 2000; 53:148-54. doi: 10.1016/s1567-424x(09)70150-3
González-Fernández M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: An overview. Current Physical Medicine and Rehabilitation Reports. 2013; 1(3):187–96. doi: 10.1007/s40141-013-0017-y
Logemann JA. Swallowing disorders. Best practice & research Clinical gastroenterology. 2007; 21(4):563-73. doi: 10.1016/j.bpg.2007.03.006
O’Rourke F, Vickers K, Upton C, Chan D. Swallowing and oropharyngeal dysphagia. Clinical Medicine. 2014; 14(2):196-9. doi: 10.7861/clinmedicine.14-2-196
Malandraki G, Robbins J. Dysphagia. In: Michael PB, David CG, editors. Handbook of Clinical Neurology. Philadelphia: Elsevier. 2013;
Mistry S, Hamdy S. Neural control of feeding and swallowing. Physical Medicine and Rehabilitation Clinics of North America. 2008; 19(4):709-28. doi: 10.1016/j.pmr.2008.05.002
Bronfeld M, Bar-Gad I. Tic disorders what happens in the basal ganglia? The Neuroscientist. 2013; 19(1):101-8. doi: 10.1177/1073858412444466
Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003; 60(10):1365-8. doi: 10.1001/archneur.60.10.1365
Snell RS. Clinical neuroanatomy. Philadelphia: Lippincott Williams & Wilkins; 2010.
Herrero MT, Barcia C, Navarro J. Functional anatomy of thalamus and basal ganglia. Child’s Nervous System. 2002; 18(8):386-404. doi: 10.1007/s00381-002-0604-1
Schepp SK, Tirschwell DL, Miller RM, Longstreth W. Swallowing screens after acute stroke a systematic review. Stroke. 2012;43(3):869-71. doi: 10.1161/strokeaha.111.638254
Kim IS, Han TR. Influence of mastication and salivation on swallowing in stroke patients. Archives of Physical Medicine and Rehabilitation. 2005; 86(10):1986-90. doi: 10.1016/j.apmr.2005.05.004
Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, Lawrence S, Aloysius A, Rutherford MA, et al. Feeding and communication impairments in infants with central grey matter lesions following perinatal hypoxic–ischaemic injury. European Journal of Paediatric Neurology. 2012; 16(6):688-96. doi: 10.1016/j.ejpn.2012.05.001
Potulska A, Friedman A, Królicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism & Related Disorders. 2003; 9(6):349-53. doi: 10.1016/s1353-8020(03)00045-2
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: An event-related fMRI study. The American Journal of Physiology. 1999; 277(1):219–225. PubMed: 10409170
Sutton JP. Dysphagia in Parkinson’s disease is responsive to levodopa. Parkinsonism & Related Disorders. 2013; 19(3):282-4. doi: 10.1016/j.parkreldis.2012.11.007
Michou E, Baijens L, Rofes L, Cartgena PS, Clavé P. Oropharyngeal swallowing disorders in Parkinson’s disease: revisited. International Journal of Speech & Language Pathology and Audiology. 2013; 1(2):76-88. doi: 10.12970/2311-1917.2013.01.02.5
Argolo N, Sampaio M, Pinho P, Melo A, Nóbrega AC. Swallowing disorders in Parkinson’s disease: Impact of lingual pumping. International Journal of Language & Communication Disorders. 2015; 50(5): 659-664. doi: 10.1111/1460-6984.12158
Crary M, Sura L, Madhavan A, Carnaby-Mann G. Dysphagia in the elderly: Management and nutritional considerations. Clinical Interventions in Aging. 2012; 7(287):98. doi: 10.2147/cia.s23404
Forster A, Samaras N, Gold G, Samaras D. Oropharyngeal dysphagia in older adults: A review. European Geriatric Medicine. 2011; 2(6):356-62. doi: 10.1016/j.eurger.2011.08.007
Omari T, Kritas S, Cock C, Besanko L, Burgstad C, Thompson A, et al. Swallowing dysfunction in healthy older people using pharyngeal pressure‐flow analysis. Neurogastroenterology & Motility. 2014; 26(1):59-68. doi: 10.1111/nmo.12224
Kocdor P, Siegel ER, Giese R, Tulunay‐Ugur OE. Characteristics of dysphagia in older patients evaluated at a tertiary center. The Laryngoscope. 2015; 125(2):400-5. doi: 10.1002/lary.24917
Daniels SK. Neurological disorders affecting oral, pharyngeal swallowing. GI Motility Online. 2006. doi: 10.1038/gimo34
Hartnick CJ, Rudolph C, Willging JP, Holland SK. Functional magnetic resonance imaging of the pediatric swallow: Imaging the cortex and the brainstem. Laryngoscope. 2001; 111(7):1183–91. doi: 10.1097/00005537-200107000-00010
Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. Journal of Neurophysiology. 2004; 92(4):2428-93. doi: 10.1152/jn.01144.2003.
Troche MS, Brandimore AE, Foote KD, Morishita T, Chen D, Hegland KW, et al. Swallowing outcomes following unilateral STN vs. GPi surgery: A retrospective analysis. Dysphagia. 2014; 29(4):425-31. doi: 10.1007/s00455-014-9522-0
Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. Journal of Neurology. 2000;247(S5):V1-V15. doi: 10.1007/pl00007778
Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: Use of rabies virus to reveal multisynaptic circuits. Brain Mechanisms for the Integration of Posture and Movement. 2004; 143:447-59. doi: 10.1016/s0079-6123(03)43042-2
Haber SN. The primate basal ganglia: Parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003; 26(4):317-30. doi: 10.1016/j.jchemneu.2003.10.003
Alberts MJ, Horner J, Gray L, Brazer SR. Aspiration after stroke: lesion analysis by brain MRI. Dysphagia. 1992; 7(3):170-3. doi: 10.1007/bf02493452
Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: A systematic review. Dysphagia. 2007; 22(3):266-75. doi: 10.1007/s00455-007-9080-9
Chakravarthy VS, Joseph D, Bapi RS. What do the basal ganglia do? A modeling perspective. Biological Cybernetics. 2010; 103(3):237-53. doi: 10.1007/s00422-010-0401-y
Cunnington R, Iansek R, Bradshaw JL, Phillips JG. Movement-related potentials in Parkinson’s disease Presence and predictability of temporal and spatial cues. Brain. 1995; 118(4):935-50. doi: 10.1093/brain/118.4.935
Suntrup S, Warnecke T, Kemmling A, Teismann IK, Hamacher C, Oelenberg S, et al. Dysphagia in patients with acute striatocapsular hemorrhage. Journal of Neurology. 2011; 259(1):93-9. doi: 10.1007/s00415-011-6129-3
Grasso M, Mazzini L, Schieppati M. Muscle relaxation in Parkinson’s disease: A reaction time study. Movement Disorders. 1996; 11(4):411-20. doi: 10.1002/mds.870110410
Leopold NA, Kagel MC. Dysphagia—ingestion or deglutition?: A proposed paradigm. Dysphagia. 1997; 12(4):202-6. doi: 10.1007/pl00009537
Prosiegel M. Neurology of swallowing and dysphagia. medical radiology. Dysphagia: Springer. 2012; 83-106. doi: 10.1007/174_2011_339
Kern MK, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. American Journal of Physiol ogy. Gastrointest Liver Physiology. 2001; 280(4):531–538. PubMed: 11254478
Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of Neurophysiol. 2001; 85(2):938–950. PubMed: 11160524
Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008; 42(1):285–295. doi: 10.1016/j.neuroimage.2008.04.234
Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: An attempt to separate the components of deglutition. Human Brain Mapping. 2009; 30(10):3209–3226. doi: 10.1002/hbm.2074
Soros P, Lalone E, Smith R, Stevens T, Theurer J, Menon RS, Martin RE. Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience. 2008; 153(4):1300–1308. doi: 10.1016/j.neuroscience.2008.02.079
Swanson LW. Cerebral hemisphere regulation of motivated behaviour. Brain Research. 2000; 886(1-2):113–164. doi: 10.1016/s0006-8993(00)02905-x
Grillner S, Georgopoulos P, Jordan LM (1997). Selection and initiation of motor behavior. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Networks and Motor Behavior. Cambridge: MIT Press; 1997.
Hikosaka O, Takikawa Y & Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews. 2000; 80(3):953–978. PMID: 10893428
Takakusaki K, Saitoh K, Harada H & Kashiwayanagi M. Role of basal ganglia–brainstem pathways in the control of motor behaviors. Neuroscience Research. 2004; 50(2):137–151. doi: 10.1016/j.neures.2004.06.015
M´enard A, Auclair F, Bourcier-Lucas C, Grillner S & Dubuc R. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. The Journal of Comparative Neurology. 2007; 501(2):260–273. doi: 10.1002/cne.21258
M´enard A & Grillner S. Diencephalic locomotor region in the lamprey–afferents and efferent control. Journal of Neurophysiology. 2008; 100(3):1343–1353. doi: 10.1152/jn.01128.2007
Takakusaki K. Forebrain control of locomotor behaviors. Brain Research Reviews. 2008; 57(1):192–198. doi: 10.1016/j.brainresrev.2007.06.024
Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary Conservation of the Basal Ganglia as a Common Vertebrate Mechanism for Action Selection. Current Biology. 2011; 21(13):1081–91. doi: 10.1016/j.cub.2011.05.001
Brudzyn´ski SM, Mogenson GJ. Association of the mesencephalic locomotor region with locomotor activity induced by injections of amphetamine into the nucleus accumbens. Brain Research. 1985; 334(1):77–84. doi: 10.1016/0006-8993(85)90569-4
Grillner S, Hellgren J, M´enard A, Saitoh K, Wikstrom M. Mechanisms for selection of basic motor programs – roles for the striatum and pallidum. Trends in Neuroscience. 2005; 28(7):364–70. doi: 10.1016/j.tins.2005.05.004
Kozlov A, Huss M, Lansner A, Kotaleski JH, Grillner S. Simple cellular and network control principles govern complex patterns of motor behavior. Proceedings of the National Academy of Sciences. 2009; 106(47):20027–32. doi: 10.1073/pnas.0906722106
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986; 9(1):357–81. doi: 10.1146/annurev.ne.09.030186.002041
Bjursten LM, Norrsell K, Norrsell U. Behavioural repertory of cats without cerebral cortex from infancy. Experimental Brain Research. 1976; 25(2):115. doi: 10.1007/bf00234897
DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990; 13(7):281–5. doi: 10.1016/0166-2236(90)90110-v
Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010; 466(7306):622–6. doi: 10.1038/nature09159
Lacey CJ, Bolam JP, Magill PJ. Novel and Distinct Operational Principles of Intralaminar Thalamic Neurons and Their Striatal Projections. Journal of Neuroscience. 2007; 27(16):4374–84. doi: 10.1523/jneurosci.5519-06.2007
Doig NM, Moss J, Bolam JP. Cortical and Thalamic Innervation of Direct and Indirect Pathway Medium-Sized Spiny Neurons in Mouse Striatum. Journal of Neuroscience. 2010; 30(44):14610–8. doi: 10.1523/jneurosci.1623-10.2010
Gerfen CR, Surmeier DJ. Modulation of Striatal Projection Systems by Dopamine. Annual Review of Neuroscience. 2011; 34(1):441–66. doi: 10.1146/annurev-neuro-061010-113641
Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nature Reviews Neuroscience. 2006; 7(12):967–75. doi: 10.1038/nrn2022
Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007; 30(1):259–88. doi: 10.1146/annurev.neuro.28.061604.13572
Hikosaka O. The habenula: From stress evasion to value-based decision-making. Nature Reviews Neuroscience. 2010; 11(7):503–13. doi: 10.1038/nrn2866
Habel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to thelateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012; 74(3):475–81. doi: 10.1016/j.neuron.2012.02.037
Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. The Journal of Comparative Neurology. 2012; 520(13):2957–73. doi: 10.1002/cne.23087
Ericsson J. Cellular and synaptic properties in the lamprey striatum. [PhD thesis]. Sweden: Karolinska Institutet; 2012
Dziewas R, Sörös P, Ishii R, Chau W, Henningsen H, Ringelstein E., et al. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. NeuroImage. 2003; 20(1): 135–44. doi: 10.1016/s1053-8119(03)00285-4
4. Discussion
Swallowing is a multi-faceted performance with active neural synchronization at the cerebral and brainstem levels. Studies on functional magnetic resonance imaging (fMRI) have recognized the anatomic sections, which are active during swallowing, including the chief sensory and motor cortex, additional motor area (SMA), cingulate cortex, insula, operculum, prefrontal and inferior frontal cortices, BG, thalamus, and cerebellum [43-48]. As mentioned earlier, automatic movements of swallowing are controlled by BG that establishes accurate timing and spacing in this process [37, 38].
Although the rhythmic pattern of mastication is controlled by the central pattern generator in the brainstem, it is supplemented by the motor cortex that provides pre-programmed movement patterns based on expectations and sensory feedbacks in conjunction with BG. The production level of BG includes active GABAergic neurons arising from two nuclei, GPi and SNr. The sub-populations of GABAergic neurons from the structure have outstanding projections to diverse motor cores in the brainstem [49]. As these neurons are tonically energetic at rest, they can sustain the incessant inhibitory drive [50-56].
Therefore, starting a motor program similar to swallowing depends on the elimination of such tonic reticence; hence, the pallidal output neurons must be inhibited from the input layer of BG [51, 53, 57-59]. Beyond the pallidal control of motor centers, the neurons projected by pallidum returns to the groups of cells inside the thalamus and are further projected back to the cortex. The pallido-thalamo-cortical loop controls emotions, swallowing, and motor and cognitive functions [60]. It is worth mentioning that in neurological diseases like Parkinson’s, the cortex is out of the loop, and hence, all the actions of BG are done straightforwardly over brainstem targets [61].
It is also essential to review the BG-nuclei that manage the output level as discussed in previous studies. As demonstrated in Figure 2, the projection neurons in the striatum are divided into two groups. Firstly are the dopamine receptors D1 type (D1R) that project straightforwardly to subpopulations of neurons at the production level (SNr/GPi) and get involved in the beginning of motor programs of swallowing, and secondly are the dopamine D2 receptors (D2R) that project to GPe - a neural structure that contributes with STN and restrain movements [61-63].
The subpopulation of D1R projected neurons control the fundamental aspects of motor performance of swallowing and depend on the excitatory inputs from thalamus and cortex/pallium, which further verifies whether they are activated or not. In case activated, the D1R neurons will participate in the instigation of a given motor program for swallowing [64, 65]. The tonic level of dopamine discharge resolves the responsiveness of the striatal neurons, and thus, the negligible dopamine makes it intricate to activate the movements, similar to that observed in Parkinson’s disease [66]. Dopamine neurons of BG have another characteristic importance in the motor system, i.e., they react with short-lasting bursts of activity throughout the awareness - a trait that can be important in promoting the knowledge of swallowing behavior [67, 68]. Despite their significance, our understanding of neural circuits, which are responsible for the value-based changes in dopamine discharge, is not complete [69-71].
It is, therefore, important to mention the dissimilar components inside the BG-controlled special motor programs. The swallowing process depends on the input from pallium/cortex, thalamus and the dopamine system and includes various parts of both direct (D1) and indirect pathways (D2). The production cells from GPi and SNr aim at the diverse motor centers [55, 71]. The selection of specific units depends on the excitatory input from thalamus and pallium/cortex along with the degree of tonic dopamine activity, which collectively define the prototype of BG’s behavior. In addition varied other motor patterns can be shared, for instance, one can swallow and chew simultaneously while one can only turn left or right and not both at the same time [59].
Therefore, mechanisms must be discovered for governing different behaviors of BG. Nevertheless, it is obvious that the BG plays a key role in making a flat series of movements [65]. Therefore, movement skills are compromised in patients with Parkinson’s disease, and there is a propensity to carry out merely one motor pattern at a time [72].
In this review study, we aimed at clarifying the role of the BG in the swallowing process for the first time. Reviewing of the published data on neurological control in swallowing process revealed that BG is one of the most complicated neurological structures, partially due to its location in the brain with indistinct performances. On the other hand, few published studies have focused on neurological aspects of swallowing, indicating that it is a sophisticated process with several unknown aspects. However, with the help of neuroimaging techniques, it has been confirmed that BG is linked to neural structures that support motor and cognitive functions such as the one involved in swallowing. BG receives input from all the cortical areas and projects to prefrontal, pre-motor, and supplementary motor areas through the thalamus. In BG-thalamo-cortical circuits, the thalamus conveys the information about the sensation of eating and swallowing to other structures while BG monitors the movement of progression to ensure the accuracy of swallowing from its different aspects.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to thank Mr. Mohsen Ghiasi for designing the graphics of the article.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clinical Neurophysiology. 2003; 114(12):2226-44. doi: 10.1016/s1388-2457(03)00237-2
Ertekin C. Physiological and pathological aspects of oropharyngeal swallowing. Movement Disorders. 2002; 17(S2):S86–S89. doi: 10.1002/mds.10068
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: Normal and abnormal. Physical Medicine and Rehabilitation Clinics of North America. 2008; 19(4):691–707. doi: 10.1016/j.pmr.2008.06.001
Jones B. Normal and abnormal swallowing: Imaging in diagnosis and therapy. Philadelphia: Springer; 2003.
Bakheit AMO. Management of neurogenic dysphagia. Postgraduate Medical Journal. 2001; 7(913):694–9. doi: 10.1136/pmj.77.913.694
Ertekin C, Palmer JB. Chapter 19 Physiology and electromyography of swallowing and its disorders. Supplements to Clinical Neurophysiology. 2000; 53:148-54. doi: 10.1016/s1567-424x(09)70150-3
González-Fernández M, Ottenstein L, Atanelov L, Christian AB. Dysphagia after stroke: An overview. Current Physical Medicine and Rehabilitation Reports. 2013; 1(3):187–96. doi: 10.1007/s40141-013-0017-y
Logemann JA. Swallowing disorders. Best practice & research Clinical gastroenterology. 2007; 21(4):563-73. doi: 10.1016/j.bpg.2007.03.006
O’Rourke F, Vickers K, Upton C, Chan D. Swallowing and oropharyngeal dysphagia. Clinical Medicine. 2014; 14(2):196-9. doi: 10.7861/clinmedicine.14-2-196
Malandraki G, Robbins J. Dysphagia. In: Michael PB, David CG, editors. Handbook of Clinical Neurology. Philadelphia: Elsevier. 2013;
Mistry S, Hamdy S. Neural control of feeding and swallowing. Physical Medicine and Rehabilitation Clinics of North America. 2008; 19(4):709-28. doi: 10.1016/j.pmr.2008.05.002
Bronfeld M, Bar-Gad I. Tic disorders what happens in the basal ganglia? The Neuroscientist. 2013; 19(1):101-8. doi: 10.1177/1073858412444466
Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Archives of neurology. 2003; 60(10):1365-8. doi: 10.1001/archneur.60.10.1365
Snell RS. Clinical neuroanatomy. Philadelphia: Lippincott Williams & Wilkins; 2010.
Herrero MT, Barcia C, Navarro J. Functional anatomy of thalamus and basal ganglia. Child’s Nervous System. 2002; 18(8):386-404. doi: 10.1007/s00381-002-0604-1
Schepp SK, Tirschwell DL, Miller RM, Longstreth W. Swallowing screens after acute stroke a systematic review. Stroke. 2012;43(3):869-71. doi: 10.1161/strokeaha.111.638254
Kim IS, Han TR. Influence of mastication and salivation on swallowing in stroke patients. Archives of Physical Medicine and Rehabilitation. 2005; 86(10):1986-90. doi: 10.1016/j.apmr.2005.05.004
Martinez-Biarge M, Diez-Sebastian J, Wusthoff CJ, Lawrence S, Aloysius A, Rutherford MA, et al. Feeding and communication impairments in infants with central grey matter lesions following perinatal hypoxic–ischaemic injury. European Journal of Paediatric Neurology. 2012; 16(6):688-96. doi: 10.1016/j.ejpn.2012.05.001
Potulska A, Friedman A, Królicki L, Spychala A. Swallowing disorders in Parkinson’s disease. Parkinsonism & Related Disorders. 2003; 9(6):349-53. doi: 10.1016/s1353-8020(03)00045-2
Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: An event-related fMRI study. The American Journal of Physiology. 1999; 277(1):219–225. PubMed: 10409170
Sutton JP. Dysphagia in Parkinson’s disease is responsive to levodopa. Parkinsonism & Related Disorders. 2013; 19(3):282-4. doi: 10.1016/j.parkreldis.2012.11.007
Michou E, Baijens L, Rofes L, Cartgena PS, Clavé P. Oropharyngeal swallowing disorders in Parkinson’s disease: revisited. International Journal of Speech & Language Pathology and Audiology. 2013; 1(2):76-88. doi: 10.12970/2311-1917.2013.01.02.5
Argolo N, Sampaio M, Pinho P, Melo A, Nóbrega AC. Swallowing disorders in Parkinson’s disease: Impact of lingual pumping. International Journal of Language & Communication Disorders. 2015; 50(5): 659-664. doi: 10.1111/1460-6984.12158
Crary M, Sura L, Madhavan A, Carnaby-Mann G. Dysphagia in the elderly: Management and nutritional considerations. Clinical Interventions in Aging. 2012; 7(287):98. doi: 10.2147/cia.s23404
Forster A, Samaras N, Gold G, Samaras D. Oropharyngeal dysphagia in older adults: A review. European Geriatric Medicine. 2011; 2(6):356-62. doi: 10.1016/j.eurger.2011.08.007
Omari T, Kritas S, Cock C, Besanko L, Burgstad C, Thompson A, et al. Swallowing dysfunction in healthy older people using pharyngeal pressure‐flow analysis. Neurogastroenterology & Motility. 2014; 26(1):59-68. doi: 10.1111/nmo.12224
Kocdor P, Siegel ER, Giese R, Tulunay‐Ugur OE. Characteristics of dysphagia in older patients evaluated at a tertiary center. The Laryngoscope. 2015; 125(2):400-5. doi: 10.1002/lary.24917
Daniels SK. Neurological disorders affecting oral, pharyngeal swallowing. GI Motility Online. 2006. doi: 10.1038/gimo34
Hartnick CJ, Rudolph C, Willging JP, Holland SK. Functional magnetic resonance imaging of the pediatric swallow: Imaging the cortex and the brainstem. Laryngoscope. 2001; 111(7):1183–91. doi: 10.1097/00005537-200107000-00010
Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, et al. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: A functional magnetic resonance imaging study. Journal of Neurophysiology. 2004; 92(4):2428-93. doi: 10.1152/jn.01144.2003.
Troche MS, Brandimore AE, Foote KD, Morishita T, Chen D, Hegland KW, et al. Swallowing outcomes following unilateral STN vs. GPi surgery: A retrospective analysis. Dysphagia. 2014; 29(4):425-31. doi: 10.1007/s00455-014-9522-0
Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. Journal of Neurology. 2000;247(S5):V1-V15. doi: 10.1007/pl00007778
Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: Use of rabies virus to reveal multisynaptic circuits. Brain Mechanisms for the Integration of Posture and Movement. 2004; 143:447-59. doi: 10.1016/s0079-6123(03)43042-2
Haber SN. The primate basal ganglia: Parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003; 26(4):317-30. doi: 10.1016/j.jchemneu.2003.10.003
Alberts MJ, Horner J, Gray L, Brazer SR. Aspiration after stroke: lesion analysis by brain MRI. Dysphagia. 1992; 7(3):170-3. doi: 10.1007/bf02493452
Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: A systematic review. Dysphagia. 2007; 22(3):266-75. doi: 10.1007/s00455-007-9080-9
Chakravarthy VS, Joseph D, Bapi RS. What do the basal ganglia do? A modeling perspective. Biological Cybernetics. 2010; 103(3):237-53. doi: 10.1007/s00422-010-0401-y
Cunnington R, Iansek R, Bradshaw JL, Phillips JG. Movement-related potentials in Parkinson’s disease Presence and predictability of temporal and spatial cues. Brain. 1995; 118(4):935-50. doi: 10.1093/brain/118.4.935
Suntrup S, Warnecke T, Kemmling A, Teismann IK, Hamacher C, Oelenberg S, et al. Dysphagia in patients with acute striatocapsular hemorrhage. Journal of Neurology. 2011; 259(1):93-9. doi: 10.1007/s00415-011-6129-3
Grasso M, Mazzini L, Schieppati M. Muscle relaxation in Parkinson’s disease: A reaction time study. Movement Disorders. 1996; 11(4):411-20. doi: 10.1002/mds.870110410
Leopold NA, Kagel MC. Dysphagia—ingestion or deglutition?: A proposed paradigm. Dysphagia. 1997; 12(4):202-6. doi: 10.1007/pl00009537
Prosiegel M. Neurology of swallowing and dysphagia. medical radiology. Dysphagia: Springer. 2012; 83-106. doi: 10.1007/174_2011_339
Kern MK, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. American Journal of Physiol ogy. Gastrointest Liver Physiology. 2001; 280(4):531–538. PubMed: 11254478
Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. Journal of Neurophysiol. 2001; 85(2):938–950. PubMed: 11160524
Lowell SY, Poletto CJ, Knorr-Chung BR, Reynolds RC, Simonyan K, Ludlow CL. Sensory stimulation activates both motor and sensory components of the swallowing system. Neuroimage. 2008; 42(1):285–295. doi: 10.1016/j.neuroimage.2008.04.234
Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: An attempt to separate the components of deglutition. Human Brain Mapping. 2009; 30(10):3209–3226. doi: 10.1002/hbm.2074
Soros P, Lalone E, Smith R, Stevens T, Theurer J, Menon RS, Martin RE. Functional MRI of oropharyngeal air-pulse stimulation. Neuroscience. 2008; 153(4):1300–1308. doi: 10.1016/j.neuroscience.2008.02.079
Swanson LW. Cerebral hemisphere regulation of motivated behaviour. Brain Research. 2000; 886(1-2):113–164. doi: 10.1016/s0006-8993(00)02905-x
Grillner S, Georgopoulos P, Jordan LM (1997). Selection and initiation of motor behavior. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Networks and Motor Behavior. Cambridge: MIT Press; 1997.
Hikosaka O, Takikawa Y & Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiological Reviews. 2000; 80(3):953–978. PMID: 10893428
Takakusaki K, Saitoh K, Harada H & Kashiwayanagi M. Role of basal ganglia–brainstem pathways in the control of motor behaviors. Neuroscience Research. 2004; 50(2):137–151. doi: 10.1016/j.neures.2004.06.015
M´enard A, Auclair F, Bourcier-Lucas C, Grillner S & Dubuc R. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. The Journal of Comparative Neurology. 2007; 501(2):260–273. doi: 10.1002/cne.21258
M´enard A & Grillner S. Diencephalic locomotor region in the lamprey–afferents and efferent control. Journal of Neurophysiology. 2008; 100(3):1343–1353. doi: 10.1152/jn.01128.2007
Takakusaki K. Forebrain control of locomotor behaviors. Brain Research Reviews. 2008; 57(1):192–198. doi: 10.1016/j.brainresrev.2007.06.024
Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary Conservation of the Basal Ganglia as a Common Vertebrate Mechanism for Action Selection. Current Biology. 2011; 21(13):1081–91. doi: 10.1016/j.cub.2011.05.001
Brudzyn´ski SM, Mogenson GJ. Association of the mesencephalic locomotor region with locomotor activity induced by injections of amphetamine into the nucleus accumbens. Brain Research. 1985; 334(1):77–84. doi: 10.1016/0006-8993(85)90569-4
Grillner S, Hellgren J, M´enard A, Saitoh K, Wikstrom M. Mechanisms for selection of basic motor programs – roles for the striatum and pallidum. Trends in Neuroscience. 2005; 28(7):364–70. doi: 10.1016/j.tins.2005.05.004
Kozlov A, Huss M, Lansner A, Kotaleski JH, Grillner S. Simple cellular and network control principles govern complex patterns of motor behavior. Proceedings of the National Academy of Sciences. 2009; 106(47):20027–32. doi: 10.1073/pnas.0906722106
Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986; 9(1):357–81. doi: 10.1146/annurev.ne.09.030186.002041
Bjursten LM, Norrsell K, Norrsell U. Behavioural repertory of cats without cerebral cortex from infancy. Experimental Brain Research. 1976; 25(2):115. doi: 10.1007/bf00234897
DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences. 1990; 13(7):281–5. doi: 10.1016/0166-2236(90)90110-v
Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010; 466(7306):622–6. doi: 10.1038/nature09159
Lacey CJ, Bolam JP, Magill PJ. Novel and Distinct Operational Principles of Intralaminar Thalamic Neurons and Their Striatal Projections. Journal of Neuroscience. 2007; 27(16):4374–84. doi: 10.1523/jneurosci.5519-06.2007
Doig NM, Moss J, Bolam JP. Cortical and Thalamic Innervation of Direct and Indirect Pathway Medium-Sized Spiny Neurons in Mouse Striatum. Journal of Neuroscience. 2010; 30(44):14610–8. doi: 10.1523/jneurosci.1623-10.2010
Gerfen CR, Surmeier DJ. Modulation of Striatal Projection Systems by Dopamine. Annual Review of Neuroscience. 2011; 34(1):441–66. doi: 10.1146/annurev-neuro-061010-113641
Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nature Reviews Neuroscience. 2006; 7(12):967–75. doi: 10.1038/nrn2022
Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007; 30(1):259–88. doi: 10.1146/annurev.neuro.28.061604.13572
Hikosaka O. The habenula: From stress evasion to value-based decision-making. Nature Reviews Neuroscience. 2010; 11(7):503–13. doi: 10.1038/nrn2866
Habel SJ, Proulx CD, Trias A, Murphy RT, Malinow R. Input to thelateral habenula from the basal ganglia is excitatory, aversive, and suppressed by serotonin. Neuron. 2012; 74(3):475–81. doi: 10.1016/j.neuron.2012.02.037
Stephenson-Jones M, Ericsson J, Robertson B, Grillner S. Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. The Journal of Comparative Neurology. 2012; 520(13):2957–73. doi: 10.1002/cne.23087
Ericsson J. Cellular and synaptic properties in the lamprey striatum. [PhD thesis]. Sweden: Karolinska Institutet; 2012
Dziewas R, Sörös P, Ishii R, Chau W, Henningsen H, Ringelstein E., et al. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. NeuroImage. 2003; 20(1): 135–44. doi: 10.1016/s1053-8119(03)00285-4
Article type: Reviews |
Received: 2016/07/6 | Accepted: 2016/09/18 | Published: 2016/12/1
Received: 2016/07/6 | Accepted: 2016/09/18 | Published: 2016/12/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |