Volume 18, Issue 3 (September 2020)
Iranian Rehabilitation Journal 2020, 18(3): 293-300 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirabzadeh A, Iranpour H R, Khorram Khorshid H R, Zare-Abdollahi D, Norouzi M, Vatankhah V. Investigation of DRD2 and HTR2A mRNA Expression in Two Therapeutic States of Antipsychotic Polypharmacy and Aripiprazole Monotherapy in the Peripheral Blood of Patients With Schizophrenia. Iranian Rehabilitation Journal 2020; 18 (3) :293-300
URL: http://irj.uswr.ac.ir/article-1-1052-en.html
URL: http://irj.uswr.ac.ir/article-1-1052-en.html
Arash Mirabzadeh1 

 , Hamid Reza Iranpour2
, Hamid Reza Iranpour2 

 , Hamid Reza Khorram Khorshid3
, Hamid Reza Khorram Khorshid3 

 , Davood Zare-Abdollahi3
, Davood Zare-Abdollahi3 

 , Mehdi Norouzi1
, Mehdi Norouzi1 

 , Venus Vatankhah *4
, Venus Vatankhah *4 




 , Hamid Reza Iranpour2
, Hamid Reza Iranpour2 

 , Hamid Reza Khorram Khorshid3
, Hamid Reza Khorram Khorshid3 

 , Davood Zare-Abdollahi3
, Davood Zare-Abdollahi3 

 , Mehdi Norouzi1
, Mehdi Norouzi1 

 , Venus Vatankhah *4
, Venus Vatankhah *4 


1- Social Determinants of Health Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Department of Molecular Biology, Pasteur Institute of Iran, Tehran, Iran.
3- Genetics Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Imam Khomeini Hospital Complex of Firuzkuh, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Molecular Biology, Pasteur Institute of Iran, Tehran, Iran.
3- Genetics Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Imam Khomeini Hospital Complex of Firuzkuh, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 675 kb]
(1297 Downloads)
| Abstract (HTML) (2918 Views)
Under the specific thermal conditions (95ºC for 15 min, then 45 cycles of 94ºC for 15 s, 60ºC for 25 s, and 72ºC for 15 s), the reactions were doubled. The accuracy of the amplification products was confirmed by the melting curve analysis and by the detection of exact size PCR products, using agarose 2.5% gel electrophoresis. First, qRT-PCRs were performed with 5-fold dilutions (each with 5 replicates) with reference cDNA (controls [pooled cDNA]) covering the expected detection range to get the standard curves for the target gene and HPRT. The amplification efficiencies were 0.92 for DRD2, 0.96 for HTR2A, and 0.94 for HPRT genes. Based on these curves, samples defined as calibrator and standard (controls [pooled cDNA]) had been included in each run. The 2-ddCt value was used to compare the relative expression of genes between two investigated groups (before and after the treatment).
Data analysis
The Mean±SD were reported for quantitative variables and the frequency table for qualitative variables. The obtained data were analyzed using the correlation test, paired t-test [17], and Wilcoxon test [18]. All analyses were done in SPSS v. 16.0.
3. Results
In this study, a total of 26 patients with schizophrenia who met the inclusion criteria were investigated. Seven patients (4 women and 3 men) were excluded from the study during the intervention. The exclusion reasons were as follows:
● Patient’s permanent discharge from the hospital;
● The patient was unwilling to continue the study in the first week of intervention;
● Escaping from the hospital in the fourth week;
● Patient’s uncontrolled diabetes in the fourth week of the study; and
● Exacerbation of schizophrenia symptoms in the form of psychosis relapse and agitation during the 9th-11th week of the study period.
Of the remaining 19 patients in the study, 15 patients (78.9%) were men and 4 patients (21.1%) were women.
The Mean±SD age of the studied patients was 49±9.49 years with a minimum age of 33 and a maximum age of 65 years. The Mean±SD age of the male patients was 50.46±7.43 years with the minimum 38 and maximum of 60 years, and the Mean±SD age of the female patients was 45.33±12.72 years with the minimum age of 33 and maximum age of 65 years.
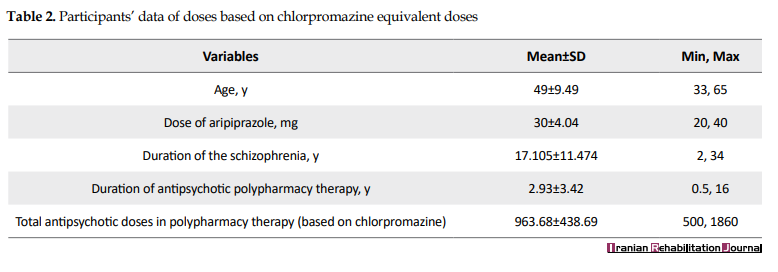
The Mean±SD of duration of their disorder was 17.105±11.474 years with a range of 2-34 years. The Mean±SD of duration of using antipsychotic polypharmacy was 3.42±2.93 years with a range of 0.5-16 years. Additionally, the Mean±SD of total dose of the received antipsychotic polypharmacy was 936.68±438.69 mg with a range of 500-1860 mg based on the chlorpromazine equivalent dosage before and after the intervention (Table 2).
DRD2 expression changes were measured using the paired t-test. Based on the test results, its changes had significantly reduced after substituting the patient’s antipsychotic medication from polypharmacy state to aripiprazole monotherapy (P=0.044) (Figure 1).
Considering that the data were not distributed normally, the Wilcoxon non-parametric test was used to determine the HTR2A expression rate. Although the HTR2A expression rate decreased after the intervention, this reduction was not significant (P=0.32) (Figures 2 & 3).
.PNG)
In patients whose polypharmacy duration was less than 24 months, DRD2 expression changes were accompanied by a significant reduction (P=0.02), but HTR2A expression changes were not significant (P≥0.05).
In patients whose treatment duration was more than 24 months, HTR2A expression (P=0.6) and DRD2 expression changes (P=0.4) were not significant. In patients whose disease lasted less than 10 years, the DRD2 expression rate was accompanied by a significant reduction (P=0.042), but the HTR2A expression rate showed no significant change (P>0.05). Besides, no significant change was observed in DRD2 and HTR2A expression rates among patients whose disorder duration was more than 10 years.
DRD2 gene expression rate significantly reduced after aripiprazole monotherapy in patients who previously received polypharmacy with chlorpromazine equivalent dosage of 700-1000 mg (P=0.06) and chlorpromazine equivalent dosage of more than 1000 mg (P=0.012). Therefore, the previous antipsychotic dosage had not affected the DRD2 expression level.
The Mean±SD) BPRS score of patients in the basic state is 25.736±10.521 and the Mean±SD BPRS score after the intervention was 26.6±12 and no observed significant changes in BPRS score between 2 states. Additionally, no significant relationship was observed between BPRS changes and DRD2 and HTR2A expression changes before and after the intervention (P≥0.05).
4. Discussion
Schizophrenia is one of the most serious psychiatric disorders. Considering the studies conducted on this disease, the dopaminergic and serotoninergic hypothesis cannot describe all symptoms of schizophrenia [19]. Four studies indicate the lack of notable differences in the mRNA expression levels of DRD2 in patients with schizophrenia and the control group. Of these studies, 3 studies were conducted on the peripheral blood sample [7, 20, 21] and one study on postmortal prefrontal cortex samples [3]. Two investigations reported that the mRNA expression level of DRD2 in the blood samples of schizophrenia patients is higher than that in the control group [6, 22]. Another study reported the mRNA expression level of DRD2 was low in the blood samples of patients with schizophrenia and its related disorders during the high delusion state [6]. These contradictory results were obtained because schizophrenia includes a group of highly heterogeneous syndromes. Schizophrenia spectrum patients show very different symptoms [23]. The mRNA expression level of DRD2 in the peripheral blood may indicate the psychiatric symptoms, and may not be a specific indicator related to schizophrenia itself [7]. Our data show that the mRNAs expression level of DRD2 in patients with schizophrenia has significantly reduced after treating with aripiprazole monotherapy compared to polypharmacy state. A large number of studies indicate that the HT2A receptor is one of the most important targets of atypical antipsychotics. In a study, no significant relationship was found between HTR2A and the effectiveness of risperidone [24].
DRD2, HTR2A, and HTR2C are important genes in the pharmacodynamics of risperidone and several studies have shown the importance of these genes in the antipsychotic responses [25]. In the current research, DRD2 and HTR2A genes expression changes in the peripheral blood of patients were compared in 2 states: antipsychotic polypharmacy and aripiprazole monotherapy. It was found that the DRD2 gene expression rate was accompanied by a significant reduction after the intervention, but the HTR2A gene expression rate showed no significant changes. In this study, DRD2 gene expression changes were significantly reduced in patients whose polypharmacy duration was less than 24 months, but in the group with polypharmacy duration of more than 24 months, no significant changes were observed. Additionally, in patients whose disorder duration was less than 10 years, the DRD2 gene expression changes were significant, but in patients with schizophrenia disorder duration of more than 10 years, no significant difference was observed regarding the DRD2 gene expression level. These observations can indicate that changing polypharmacy state to aripiprazole monotherapy has caused a reduction in DRD2 gene expression only in patients with shorter disorder duration, and perhaps treatment in this group is accompanied by better clinical results. In this study, the amount of response to treatment was also investigated based on the reduction of clinical symptoms in the BPRS questionnaire.
Before the intervention, the Mean±SD BPRS score of patients was 25.736±10.521, which changed to 26.6±12 after the treatment, but this change is not statistically significant. Moreover, no significant relationship was observed between BPRS score changes and DRD2 and HTR2A gene expression levels before and after the study. Lack of reduction in clinical symptoms based on the BPRS test can be due to the short duration of study to investigate the symptoms changes. In 2015, Mirabzadeh et al. conducted a clinical trial to investigate the consumption pattern variation of antipsychotic polypharmacy to risperidone monotherapy in old patients with chronic schizophrenia. Their results showed a significant reduction in the mean of total and positive symptoms of schizophrenia based on the BPRS test following treatment with risperidone [26]. The difference between the results of our study and the above-mentioned research in the reduction of patients’ symptoms following the change of therapeutic diet to aripiprazole monotherapy can be because of the different age groups of patients in both groups. The elderly people seem to be more sensitive to medication-related side effects of antipsychotic polypharmacy and following changes to antipsychotic monotherapy state show a significant reduction in the score of BPRS test.
In this study, we had limitations in the follow up of the patients in the antipsychotic monotherapy state. This can affect the lack of significant changes in the results of the BPRS test. It is proposed that similar studies be conducted with longer follow-up durations. In this study, the number of female patients who met the inclusion criteria was less than the number of male patients, leading to the reduction of the validity of the obtained results from variables based on the patients’ gender. It is suggested that in the future, studies be conducted with larger sample size and appropriate gender distribution. The studied population in this research consisted of schizophrenic patients hospitalized in long-stay inpatient wards of a psychiatric hospital, and often a long time had passed since the onset of the disorder in them, so they had received antipsychotic therapy for a long time. The results obtained from this study cannot be generalized to all psychiatric patients of the society, therefore, it is suggested that another study be conducted to investigate the gene expression changes in patients with acute schizophrenia.
5. Conclusion
By determining the consumption pattern of antipsychotic medications in patients with chronic schizophrenia and showing how treatment type is related to the studied variables, besides using the appropriate pattern of treating patients, we can use the difference in the DRD2 gene expression to follow up the treatment of these patients.
According to this study, DRD2 gene expression is an appropriate parameter to investigate the amount of response to treatment in schizophrenic patients, which needs further studies with a larger sample size to be approved. Changes in DRD2 and HTR2A genes expression level showed no significant relationship with the amount of response to the patients’ treatment based on the BPRS questionnaire.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of the University of Social Welfare and Rehabilitation Sciences (IR.USWR.REC.1396.154) and the National Institute for Medical Research Development (IR.NIMAD.REC.1398.059).
Funding
The present article was financially supported by the National Institute for Medical Research Development (grants No.: 973020).
Authors' contributions
Methodology: Venus Vatankhah, Hamidreza Iranpour, Mehdi Noroozi, Hamid Reza Khorram Khorshid, and Davood Zare-Abdollahi; Investigation: Ararsh Mirabzadeh and Venus Vatankhah; Writing - original draft: Venus Vatankhah and Hamidreza Iranpour; Writing - review & editing: Ararsh Mirabzadeh and Mehdi Noroozi; Funding acquisition: Ararsh Mirabzadeh, Venus Vatankhah, and Hamidreza Iranpoor; Supervision: Arash Mirabzadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
This research was done at the University of Social Welfare and Rehabilitation Sciences and Razi Psychiatry Hospital, Tehran, Iran. We thank our patients that cooperated with us to change their medications.
References
Full-Text: (1006 Views)
1. Introduction
Considering the existence of a major regulatory mechanism in which various molecular factors are involved, the pathophysiological complexity of schizophrenia and its psychiatric disorders pose important questions in this regard [1]. Continuous advances in genomic and molecular biology create new opportunities to develop our perception of cellular mechanisms, schizophrenia development, and antipsychotic medications by investigating their effect on cell signaling [2].
Dopamine D2 receptor (DRD2) and 5-hydroxytryptamine 2A Receptor (HTR2A) are the key elements of dopaminergic and serotoninergic systems, respectively [3, 4]. The analysis of mRNA expression of dopamine receptors in the peripheral blood is a useful tool to assess the mechanisms of dopaminergic function, which is based on the complicated changes in the psychological characteristics and psychopathology of psychiatric diseases [5]. In the microarray analysis, dopamine receptor mRNA was overexpressed in the peripheral blood of schizophrenic patients who took medication [6]. This receptor mRNA level in peripheral blood indicates acute schizophrenia [7].
HTR2A receptor affects prefrontal perception by binding to the agonist receptor, including quetiapine and 3-trifluoromethylphenylpiperazine [8]. It has been suggested that HTR2A antagonists are used as a treatment to improve cognitive performance, though the effectiveness of HTR2A antagonists has not been proven so far [9].
In all schizophrenia therapeutic guidelines, treatment with single-drug therapy is preferable, and as the last solution, the use of two or more antipsychotic medications is recommended [10, 11]. The antipsychotic treatment with multi-drug therapy is often used experimentally, and the prevalence of multi-drug therapy in the world is about 40%-70%, which differs based on the patients’ population and the existing conditions in different countries. However, it has been on the rise over recent years [12, 13]. Multi-drug therapy using antipsychotic medications result in overdose or excessive consumption of antipsychotic drugs in patients and consequently lead to dose-related adverse effects, including extrapyramidal side-effects and cognitive problems [14].
Considering the unique mechanism of aripiprazole as monotherapy, this drug reduces the metabolic side effects of antipsychotic polypharmacy and can partially or completely improve the increased level of prolactin and metabolic side effects [15].
By measuring dopamine and serotonin receptor mRNA expression rate to follow up the treatment of schizophrenia in this study, the response to treatment is investigated in 2 states of antipsychotic polypharmacy and aripiprazole monotherapy, and then, the results will be compared with changes in BPRS score.
2. Methods
This study was a clinical trial study conducted in the form of comparison before and after intervention in the case group (patients with schizophrenia). Of all patients with chronic schizophrenia (more than 2 years) in all long-term care wards of Razi Psychiatric Hospital, all chronic schizophrenic patients taking at least two types of antipsychotic medications with a total dosage equal to or more than 500 mg chlorpromazine for more than 6 months were included in the study. At the beginning of the study, the patients’ age, gender, the number and type of all psychiatric drugs, especially antipsychotic medications, as well as their chlorpromazine equivalent dosage, total duration of disease, and duration of antipsychotic polypharmacy were determined. According to statistics computations, 26 patients were included in the study. Before starting the intervention, the score of the Brief Psychiatric Rating Scale (BPRS) test, as well as dopamine and serotonin receptor gene expression rates were measured. The intervention basis was the conversion of antipsychotic polypharmacy to aripiprazole monotherapy. In the first stage, the equivalent dosage of chlorpromazine was determined. Each 100 mg chlorpromazine is equivalent to 7.5 mg of aripiprazole [16]. Gradually and by considering the relapse cases, the dose of previous medications was reduced and aripiprazole with a dose of 2.5 mg was started. Finally, the aripiprazole dosage reached the calculated total dosage and the given maximum dosage was determined for medication. After reaching the aripiprazole monotherapy state, the treatment continues for 6 months, and during this period, the patients were visited regularly by a psychiatrist in terms of clinical symptoms and side effects. BPRS test score, as well as dopamine and serotonin receptor mRNA expression rate, were measured in 2 states: Antipsychotic polypharmacy and 6 months after aripiprazole monotherapy and the results were compared with each other.
Inclusion criteria
The included patients must be 18-65 years old with chronic schizophrenia (more than 2 years) who received at least two types of antipsychotic medications with a dosage of more than 500 mg chlorpromazine per day and were consent to participate in the study.
Exclusion criteria
The study patients would be excluded if they showed any signs of autoimmune diseases, uncontrolled diabetes, cardiovascular diseases, a severe infectious disease which requires serious intervention, using immunosuppressive therapy, the existence of other severe brain diseases such as seizures and multiple sclerosis, the existence of mental disability, alcohol or drug abuse, co-existence of other major psychiatric diseases that require drug therapy, exacerbation of disorder symptoms that are inconsistent with continuing clinical trials, and dissatisfaction to continue the study.
Data collection method
The statistical population of this study consisted of all patients who met the inclusion criteria (a total of 38 patients). The sample size was calculated to be 13, based on Lawrence S Kegeles et al.’s study, the confidence interval of 95%, the statistical power of 90%, mean of difference of 4.1, standard deviation of 4.3, 15% dropout rate and using the formula of mean comparison before and after the intervention. Given the possibility of more subjects drop out, 26 patients were included in the study.
The number of samples was estimated by the formula of mean comparison before and after the intervention (Formula 1).
Considering the existence of a major regulatory mechanism in which various molecular factors are involved, the pathophysiological complexity of schizophrenia and its psychiatric disorders pose important questions in this regard [1]. Continuous advances in genomic and molecular biology create new opportunities to develop our perception of cellular mechanisms, schizophrenia development, and antipsychotic medications by investigating their effect on cell signaling [2].
Dopamine D2 receptor (DRD2) and 5-hydroxytryptamine 2A Receptor (HTR2A) are the key elements of dopaminergic and serotoninergic systems, respectively [3, 4]. The analysis of mRNA expression of dopamine receptors in the peripheral blood is a useful tool to assess the mechanisms of dopaminergic function, which is based on the complicated changes in the psychological characteristics and psychopathology of psychiatric diseases [5]. In the microarray analysis, dopamine receptor mRNA was overexpressed in the peripheral blood of schizophrenic patients who took medication [6]. This receptor mRNA level in peripheral blood indicates acute schizophrenia [7].
HTR2A receptor affects prefrontal perception by binding to the agonist receptor, including quetiapine and 3-trifluoromethylphenylpiperazine [8]. It has been suggested that HTR2A antagonists are used as a treatment to improve cognitive performance, though the effectiveness of HTR2A antagonists has not been proven so far [9].
In all schizophrenia therapeutic guidelines, treatment with single-drug therapy is preferable, and as the last solution, the use of two or more antipsychotic medications is recommended [10, 11]. The antipsychotic treatment with multi-drug therapy is often used experimentally, and the prevalence of multi-drug therapy in the world is about 40%-70%, which differs based on the patients’ population and the existing conditions in different countries. However, it has been on the rise over recent years [12, 13]. Multi-drug therapy using antipsychotic medications result in overdose or excessive consumption of antipsychotic drugs in patients and consequently lead to dose-related adverse effects, including extrapyramidal side-effects and cognitive problems [14].
Considering the unique mechanism of aripiprazole as monotherapy, this drug reduces the metabolic side effects of antipsychotic polypharmacy and can partially or completely improve the increased level of prolactin and metabolic side effects [15].
By measuring dopamine and serotonin receptor mRNA expression rate to follow up the treatment of schizophrenia in this study, the response to treatment is investigated in 2 states of antipsychotic polypharmacy and aripiprazole monotherapy, and then, the results will be compared with changes in BPRS score.
2. Methods
This study was a clinical trial study conducted in the form of comparison before and after intervention in the case group (patients with schizophrenia). Of all patients with chronic schizophrenia (more than 2 years) in all long-term care wards of Razi Psychiatric Hospital, all chronic schizophrenic patients taking at least two types of antipsychotic medications with a total dosage equal to or more than 500 mg chlorpromazine for more than 6 months were included in the study. At the beginning of the study, the patients’ age, gender, the number and type of all psychiatric drugs, especially antipsychotic medications, as well as their chlorpromazine equivalent dosage, total duration of disease, and duration of antipsychotic polypharmacy were determined. According to statistics computations, 26 patients were included in the study. Before starting the intervention, the score of the Brief Psychiatric Rating Scale (BPRS) test, as well as dopamine and serotonin receptor gene expression rates were measured. The intervention basis was the conversion of antipsychotic polypharmacy to aripiprazole monotherapy. In the first stage, the equivalent dosage of chlorpromazine was determined. Each 100 mg chlorpromazine is equivalent to 7.5 mg of aripiprazole [16]. Gradually and by considering the relapse cases, the dose of previous medications was reduced and aripiprazole with a dose of 2.5 mg was started. Finally, the aripiprazole dosage reached the calculated total dosage and the given maximum dosage was determined for medication. After reaching the aripiprazole monotherapy state, the treatment continues for 6 months, and during this period, the patients were visited regularly by a psychiatrist in terms of clinical symptoms and side effects. BPRS test score, as well as dopamine and serotonin receptor mRNA expression rate, were measured in 2 states: Antipsychotic polypharmacy and 6 months after aripiprazole monotherapy and the results were compared with each other.
Inclusion criteria
The included patients must be 18-65 years old with chronic schizophrenia (more than 2 years) who received at least two types of antipsychotic medications with a dosage of more than 500 mg chlorpromazine per day and were consent to participate in the study.
Exclusion criteria
The study patients would be excluded if they showed any signs of autoimmune diseases, uncontrolled diabetes, cardiovascular diseases, a severe infectious disease which requires serious intervention, using immunosuppressive therapy, the existence of other severe brain diseases such as seizures and multiple sclerosis, the existence of mental disability, alcohol or drug abuse, co-existence of other major psychiatric diseases that require drug therapy, exacerbation of disorder symptoms that are inconsistent with continuing clinical trials, and dissatisfaction to continue the study.
Data collection method
The statistical population of this study consisted of all patients who met the inclusion criteria (a total of 38 patients). The sample size was calculated to be 13, based on Lawrence S Kegeles et al.’s study, the confidence interval of 95%, the statistical power of 90%, mean of difference of 4.1, standard deviation of 4.3, 15% dropout rate and using the formula of mean comparison before and after the intervention. Given the possibility of more subjects drop out, 26 patients were included in the study.
The number of samples was estimated by the formula of mean comparison before and after the intervention (Formula 1).
RNA isolation and reverse transcription and quantitative reverse transcription-polymerase chain reaction (qRT-PCR):
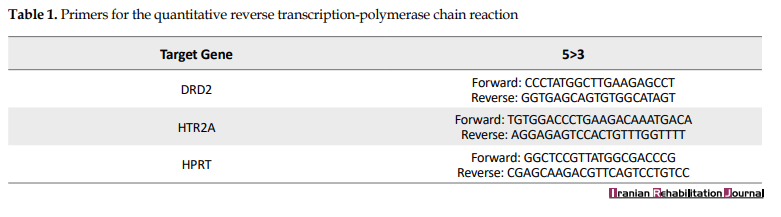
Mononuclear cells have been separated on Ficoll-Hypaque from pre-treatment and post-treatment blood samples. Total RNA extraction was carried out through TRIzol reagent (Bioneer, Daejeon, Republic of Korea). Complementary DNA (cDNA) has been synthesized with RevertAid First Strand cDNA Synthesis Kit (Fermentas, USA). qRT-PCR (Corbett 6000) was conducted for DRD2, HTR2A, and HPRT. Also, the Maxima SYBR Green/ROX qPCR as a Master Mix (Fermentas) was utilized. The relative measurement of the targeted genes (DRD2 and HTR2A) expression in the samples before and after the therapeutic intervention was gotten by normalizing to HPRT, as an endogenous control. We have developed a qRT-PCR measure utilizing primer pairs to detect targeted transcripts sensitively (Table 1) and approved on a control pooled cDNA.
Mononuclear cells have been separated on Ficoll-Hypaque from pre-treatment and post-treatment blood samples. Total RNA extraction was carried out through TRIzol reagent (Bioneer, Daejeon, Republic of Korea). Complementary DNA (cDNA) has been synthesized with RevertAid First Strand cDNA Synthesis Kit (Fermentas, USA). qRT-PCR (Corbett 6000) was conducted for DRD2, HTR2A, and HPRT. Also, the Maxima SYBR Green/ROX qPCR as a Master Mix (Fermentas) was utilized. The relative measurement of the targeted genes (DRD2 and HTR2A) expression in the samples before and after the therapeutic intervention was gotten by normalizing to HPRT, as an endogenous control. We have developed a qRT-PCR measure utilizing primer pairs to detect targeted transcripts sensitively (Table 1) and approved on a control pooled cDNA.
Under the specific thermal conditions (95ºC for 15 min, then 45 cycles of 94ºC for 15 s, 60ºC for 25 s, and 72ºC for 15 s), the reactions were doubled. The accuracy of the amplification products was confirmed by the melting curve analysis and by the detection of exact size PCR products, using agarose 2.5% gel electrophoresis. First, qRT-PCRs were performed with 5-fold dilutions (each with 5 replicates) with reference cDNA (controls [pooled cDNA]) covering the expected detection range to get the standard curves for the target gene and HPRT. The amplification efficiencies were 0.92 for DRD2, 0.96 for HTR2A, and 0.94 for HPRT genes. Based on these curves, samples defined as calibrator and standard (controls [pooled cDNA]) had been included in each run. The 2-ddCt value was used to compare the relative expression of genes between two investigated groups (before and after the treatment).
Data analysis
The Mean±SD were reported for quantitative variables and the frequency table for qualitative variables. The obtained data were analyzed using the correlation test, paired t-test [17], and Wilcoxon test [18]. All analyses were done in SPSS v. 16.0.
3. Results
In this study, a total of 26 patients with schizophrenia who met the inclusion criteria were investigated. Seven patients (4 women and 3 men) were excluded from the study during the intervention. The exclusion reasons were as follows:
● Patient’s permanent discharge from the hospital;
● The patient was unwilling to continue the study in the first week of intervention;
● Escaping from the hospital in the fourth week;
● Patient’s uncontrolled diabetes in the fourth week of the study; and
● Exacerbation of schizophrenia symptoms in the form of psychosis relapse and agitation during the 9th-11th week of the study period.
Of the remaining 19 patients in the study, 15 patients (78.9%) were men and 4 patients (21.1%) were women.
The Mean±SD age of the studied patients was 49±9.49 years with a minimum age of 33 and a maximum age of 65 years. The Mean±SD age of the male patients was 50.46±7.43 years with the minimum 38 and maximum of 60 years, and the Mean±SD age of the female patients was 45.33±12.72 years with the minimum age of 33 and maximum age of 65 years.
The Mean±SD of duration of their disorder was 17.105±11.474 years with a range of 2-34 years. The Mean±SD of duration of using antipsychotic polypharmacy was 3.42±2.93 years with a range of 0.5-16 years. Additionally, the Mean±SD of total dose of the received antipsychotic polypharmacy was 936.68±438.69 mg with a range of 500-1860 mg based on the chlorpromazine equivalent dosage before and after the intervention (Table 2).
DRD2 expression changes were measured using the paired t-test. Based on the test results, its changes had significantly reduced after substituting the patient’s antipsychotic medication from polypharmacy state to aripiprazole monotherapy (P=0.044) (Figure 1).
Considering that the data were not distributed normally, the Wilcoxon non-parametric test was used to determine the HTR2A expression rate. Although the HTR2A expression rate decreased after the intervention, this reduction was not significant (P=0.32) (Figures 2 & 3).
.PNG)
In patients whose polypharmacy duration was less than 24 months, DRD2 expression changes were accompanied by a significant reduction (P=0.02), but HTR2A expression changes were not significant (P≥0.05).
In patients whose treatment duration was more than 24 months, HTR2A expression (P=0.6) and DRD2 expression changes (P=0.4) were not significant. In patients whose disease lasted less than 10 years, the DRD2 expression rate was accompanied by a significant reduction (P=0.042), but the HTR2A expression rate showed no significant change (P>0.05). Besides, no significant change was observed in DRD2 and HTR2A expression rates among patients whose disorder duration was more than 10 years.
DRD2 gene expression rate significantly reduced after aripiprazole monotherapy in patients who previously received polypharmacy with chlorpromazine equivalent dosage of 700-1000 mg (P=0.06) and chlorpromazine equivalent dosage of more than 1000 mg (P=0.012). Therefore, the previous antipsychotic dosage had not affected the DRD2 expression level.
The Mean±SD) BPRS score of patients in the basic state is 25.736±10.521 and the Mean±SD BPRS score after the intervention was 26.6±12 and no observed significant changes in BPRS score between 2 states. Additionally, no significant relationship was observed between BPRS changes and DRD2 and HTR2A expression changes before and after the intervention (P≥0.05).
4. Discussion
Schizophrenia is one of the most serious psychiatric disorders. Considering the studies conducted on this disease, the dopaminergic and serotoninergic hypothesis cannot describe all symptoms of schizophrenia [19]. Four studies indicate the lack of notable differences in the mRNA expression levels of DRD2 in patients with schizophrenia and the control group. Of these studies, 3 studies were conducted on the peripheral blood sample [7, 20, 21] and one study on postmortal prefrontal cortex samples [3]. Two investigations reported that the mRNA expression level of DRD2 in the blood samples of schizophrenia patients is higher than that in the control group [6, 22]. Another study reported the mRNA expression level of DRD2 was low in the blood samples of patients with schizophrenia and its related disorders during the high delusion state [6]. These contradictory results were obtained because schizophrenia includes a group of highly heterogeneous syndromes. Schizophrenia spectrum patients show very different symptoms [23]. The mRNA expression level of DRD2 in the peripheral blood may indicate the psychiatric symptoms, and may not be a specific indicator related to schizophrenia itself [7]. Our data show that the mRNAs expression level of DRD2 in patients with schizophrenia has significantly reduced after treating with aripiprazole monotherapy compared to polypharmacy state. A large number of studies indicate that the HT2A receptor is one of the most important targets of atypical antipsychotics. In a study, no significant relationship was found between HTR2A and the effectiveness of risperidone [24].
DRD2, HTR2A, and HTR2C are important genes in the pharmacodynamics of risperidone and several studies have shown the importance of these genes in the antipsychotic responses [25]. In the current research, DRD2 and HTR2A genes expression changes in the peripheral blood of patients were compared in 2 states: antipsychotic polypharmacy and aripiprazole monotherapy. It was found that the DRD2 gene expression rate was accompanied by a significant reduction after the intervention, but the HTR2A gene expression rate showed no significant changes. In this study, DRD2 gene expression changes were significantly reduced in patients whose polypharmacy duration was less than 24 months, but in the group with polypharmacy duration of more than 24 months, no significant changes were observed. Additionally, in patients whose disorder duration was less than 10 years, the DRD2 gene expression changes were significant, but in patients with schizophrenia disorder duration of more than 10 years, no significant difference was observed regarding the DRD2 gene expression level. These observations can indicate that changing polypharmacy state to aripiprazole monotherapy has caused a reduction in DRD2 gene expression only in patients with shorter disorder duration, and perhaps treatment in this group is accompanied by better clinical results. In this study, the amount of response to treatment was also investigated based on the reduction of clinical symptoms in the BPRS questionnaire.
Before the intervention, the Mean±SD BPRS score of patients was 25.736±10.521, which changed to 26.6±12 after the treatment, but this change is not statistically significant. Moreover, no significant relationship was observed between BPRS score changes and DRD2 and HTR2A gene expression levels before and after the study. Lack of reduction in clinical symptoms based on the BPRS test can be due to the short duration of study to investigate the symptoms changes. In 2015, Mirabzadeh et al. conducted a clinical trial to investigate the consumption pattern variation of antipsychotic polypharmacy to risperidone monotherapy in old patients with chronic schizophrenia. Their results showed a significant reduction in the mean of total and positive symptoms of schizophrenia based on the BPRS test following treatment with risperidone [26]. The difference between the results of our study and the above-mentioned research in the reduction of patients’ symptoms following the change of therapeutic diet to aripiprazole monotherapy can be because of the different age groups of patients in both groups. The elderly people seem to be more sensitive to medication-related side effects of antipsychotic polypharmacy and following changes to antipsychotic monotherapy state show a significant reduction in the score of BPRS test.
In this study, we had limitations in the follow up of the patients in the antipsychotic monotherapy state. This can affect the lack of significant changes in the results of the BPRS test. It is proposed that similar studies be conducted with longer follow-up durations. In this study, the number of female patients who met the inclusion criteria was less than the number of male patients, leading to the reduction of the validity of the obtained results from variables based on the patients’ gender. It is suggested that in the future, studies be conducted with larger sample size and appropriate gender distribution. The studied population in this research consisted of schizophrenic patients hospitalized in long-stay inpatient wards of a psychiatric hospital, and often a long time had passed since the onset of the disorder in them, so they had received antipsychotic therapy for a long time. The results obtained from this study cannot be generalized to all psychiatric patients of the society, therefore, it is suggested that another study be conducted to investigate the gene expression changes in patients with acute schizophrenia.
5. Conclusion
By determining the consumption pattern of antipsychotic medications in patients with chronic schizophrenia and showing how treatment type is related to the studied variables, besides using the appropriate pattern of treating patients, we can use the difference in the DRD2 gene expression to follow up the treatment of these patients.
According to this study, DRD2 gene expression is an appropriate parameter to investigate the amount of response to treatment in schizophrenic patients, which needs further studies with a larger sample size to be approved. Changes in DRD2 and HTR2A genes expression level showed no significant relationship with the amount of response to the patients’ treatment based on the BPRS questionnaire.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of the University of Social Welfare and Rehabilitation Sciences (IR.USWR.REC.1396.154) and the National Institute for Medical Research Development (IR.NIMAD.REC.1398.059).
Funding
The present article was financially supported by the National Institute for Medical Research Development (grants No.: 973020).
Authors' contributions
Methodology: Venus Vatankhah, Hamidreza Iranpour, Mehdi Noroozi, Hamid Reza Khorram Khorshid, and Davood Zare-Abdollahi; Investigation: Ararsh Mirabzadeh and Venus Vatankhah; Writing - original draft: Venus Vatankhah and Hamidreza Iranpour; Writing - review & editing: Ararsh Mirabzadeh and Mehdi Noroozi; Funding acquisition: Ararsh Mirabzadeh, Venus Vatankhah, and Hamidreza Iranpoor; Supervision: Arash Mirabzadeh.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
This research was done at the University of Social Welfare and Rehabilitation Sciences and Razi Psychiatry Hospital, Tehran, Iran. We thank our patients that cooperated with us to change their medications.
References
- Szamosi A, Kelemen O, Kéri S. Hippocampal volume and the AKT signaling system in first-episode schizophrenia. Journal of Psychiatric Research. 2012; 46(3):279-84. [DOI:10.1016/j.jpsychires.2011.12.005] [PMID]
- Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. American Journal of Psychiatry. 2009; 167(4):388-96. [DOI:10.1176/appi.ajp.2009.08121873] [PMID] [PMCID]
- Zhan L, Kerr JR, Lafuente MJ, Maclean A, Chibalina MV, Liu B, et al. Altered expression and coregulation of dopamine signalling genes in schizophrenia and bipolar disorder. Neuropathology and Applied Neurobiology. 2011; 37(2):206-19. [DOI:10.1111/j.1365-2990.2010.01128.x] [PMID]
- Aghajanian GK, Marek GJ. Serotonin model of schizophrenia: Emerging role of glutamate mechanisms. Brain Research Reviews. 2000; 31(2-3):302-12. [DOI:10.1016/S0165-0173(99)00046-6]
- Kirillova GP, Hrutkay RJ, Shurin MR, Shurin GV, Tourkova IL, Vanyukov MM. Dopamine receptors in human lymphocytes: Radioligand binding and quantitative RT-PCR assays. Journal of Neuroscience Methods. 2008; 174(2):272-80. [DOI:10.1016/j.jneumeth.2008.07.018] [PMID] [PMCID]
- Zvara Á, Szekeres G, Janka Z, Kelemen JZ, Cimmer C, Sántha M, et al. Over-expression of dopamine D2 receptor and inwardly rectifying potassium channel genes in drug-naive schizophrenic peripheral blood lymphocytes as potential diagnostic markers. Disease Markers. 2005; 21(2):61-9. [DOI:10.1155/2005/275318] [PMID] [PMCID]
- Liu L, Yuan G, Cheng Z, Zhang G, Liu X, Zhang H. Identification of the mRNA expression status of the dopamine D2 receptor and dopamine transporter in peripheral blood lymphocytes of schizophrenia patients. PLoS One. 2013; 8(9):e75259. [DOI:10.1371/journal.pone.0075259] [PMID] [PMCID]
- Harvey JA. Role of the serotonin 5-HT2A receptor in learning. Learning & Memory. 2003; 10(5):355-62. [DOI:10.1101/lm.60803] [PMID] [PMCID]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry. 2005; 10(1):79-104. [DOI:10.1038/sj.mp.4001556] [PMID]
- Canadian Psychiatric Association. Clinical practice guidelines: Treatment of schizophrenia. Canadian Journal of Psychiatry. 2005; 50(13 S1):7S-57. [PMID]
- Crismon ML, Argo TR, Bendele SD, Suppes T. Texas medication algorithm project procedural manual. Texas: Texas Department of State Health Services; 2007. http://docshare01.docshare.tips/files/2986/29863697.pdf
- Procyshyn RM, Honer WG, Wu TKY, Ko RWY, Mclsaac SA, Young AH, et al. Persistent antipsychotic polypharmacy and excessive dosing in the community psychiatric treatment setting: A review of medication profiles in 435 Canadian outpatients. The Journal of Clinical Psychiatry. 2010; 71(5):566-73. [DOI:10.4088/JCP.08m04912gre] [PMID]
- Santone G, Bellantuono C, Rucci P, Picardi A, Preti A, de Girolamo G. Patient characteristics and process factors associated with antipsychotic polypharmacy in a nationwide sample of psychiatric inpatients in Italy. Pharmacoepidemiology and Drug Safety. 2011; 20(5):441-9. [DOI:10.1002/pds.2083] [PMID]
- Sakurai H, Bies RR, Stroup ST, Keefe RSE, Rajji TK, Suzuki T, et al. Dopamine D2 receptor occupancy and cognition in schizophrenia: Analysis of the CATIE data. Schizophrenia Bulletin. 2013; 39(3):564-74. [DOI:10.1093/schbul/sbr189] [PMID] [PMCID]
- Chen CK, Huang YS, Ree SC, Hsiao CC. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010; 34(8):1495-9. [DOI:10.1016/j.pnpbp.2010.08.012] [PMID]
- Vatankhah V, Mirabzadeh A, Iranpour H, Dieji B, Norouzi M, Karimipour M, et al. Determination of changes in blood biomarker levels in antipsychotic polypharmacy and aripiprazole monotherapy in patients with long-term Schizophrenia. Iranian Rehabilitation Journal. 2019; 17(4):369-76. [DOI:10.32598/irj.17.4.369]
- Hsu H, Lachenbruch PA. Paired t-test. New Jersey: John Wiley & Sons; 2007. [DOI:10.1002/9780471462422.eoct969]
- Kruskal WH. Historical notes on the Wilcoxon unpaired two-sample test. Journal of the American Statistical Association. 1957; 52(279):356-60. [DOI:10.1080/01621459.1957.10501395]
- Zheng W, Wang H, Zeng Z, Lin J, Little PJ, Srivastava LK, et al. The possible role of the Akt signaling pathway in schizophrenia. Brain Research. 2012; 1470:145-58. [DOI:10.1016/j.brainres.2012.06.032] [PMID]
- Cui Y, Prabhu V, Nguyen T, Yadav B, Chung YC. The mRNA expression status of dopamine receptor D2, dopamine receptor D3 and DARPP-32 in T lymphocytes of patients with early psychosis. International Journal of Molecular Sciences. 2015; 16(11):26677-86. [DOI:10.3390/ijms161125983] [PMID] [PMCID]
- Yao Y, Schröder J, Karlsson H. Verification of proposed peripheral biomarkers in mononuclear cells of individuals with schizophrenia. Journal of Psychiatric Research. 2008; 42(8):639-43. [DOI:10.1016/j.jpsychires.2007.07.011] [PMID]
- Kordi-Tamandani DM, Sahranavard R, Torkamanzehi A. Analysis of association between dopamine receptor genes’ methylation and their expression profile with the risk of schizophrenia. Psychiatric Genetics. 2013; 23(5):183-7. [DOI:10.1097/YPG.0b013e328363d6e1] [PMID]
- Tandon R. Schizophrenia and other psychotic disorders in DSM-5: Clinical implications of revisions from DSM-IV. Clinical Schizophrenia & Related Psychoses. 2013; 7(1):16-9. [DOI:10.3371/CSRP.TA.032513] [PMID]
- Yan Y, Wei Z, Xiong Y, Jiang J, Huo R, Shen L, et al. Association of HTR2A polymorphisms with risperidone efficacy in Chinese Han schizophrenia patients. Klinik Psikofarmakoloji Bülteni-Bulletin of Clinical Psychopharmacology. 2015; 25(1):4-11. [DOI:10.5455/bcp.20140802124158]
- Kaur G, Gupta D, Chavan BS, Sinhmar V, Prasad R, Tripathi A, et al. Identification of genetic correlates of response to Risperidone: Findings of a multicentric schizophrenia study from India. Asian Journal of Psychiatry. 2017; 29:174-82. [DOI:10.1016/j.ajp.2017.07.026] [PMID]
- Mirabzadeh A, Khodaei M, Shemshadi H. Antipsychotic polypharmacy versus monotherapy in elderly with chronic schizophrenia: A clinical trial. European Psychiatry. 2015; 30(S1):1424. [DOI:10.1016/S0924-9338(15)31100-7]
Article type: Original Research Articles |
Subject:
Psychiatry
Received: 2019/08/2 | Accepted: 2020/08/2 | Published: 2020/09/1
Received: 2019/08/2 | Accepted: 2020/08/2 | Published: 2020/09/1
Send email to the article author