Volume 21, Issue 4 (December 2023)
Iranian Rehabilitation Journal 2023, 21(4): 711-720 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Faridi F, Ameri H, Nosratabadi M, Akhavan Hejazi S M, Thatcher R W. Effect of LORETA Z-score Neurofeedback on Language, Working Memory, and Attention in People With Aphasia. Iranian Rehabilitation Journal 2023; 21 (4) :711-720
URL: http://irj.uswr.ac.ir/article-1-1785-en.html
URL: http://irj.uswr.ac.ir/article-1-1785-en.html
Farnaz Faridi *1 

 , Hayat Ameri1
, Hayat Ameri1 

 , Masoud Nosratabadi2
, Masoud Nosratabadi2 

 , Seyed Majid Akhavan Hejazi3
, Seyed Majid Akhavan Hejazi3 

 , Robert W Thatcher4
, Robert W Thatcher4 




 , Hayat Ameri1
, Hayat Ameri1 

 , Masoud Nosratabadi2
, Masoud Nosratabadi2 

 , Seyed Majid Akhavan Hejazi3
, Seyed Majid Akhavan Hejazi3 

 , Robert W Thatcher4
, Robert W Thatcher4 


1- Department of Linguistics, Faculty of Humanities, Tarbiat Modares University, Tehran, Iran.
2- Department of Clinical Psychology, School of Behavioral Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Department of Head, Brain, and Spinal Cord Injuries, Rofeideh Hospital, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Applied Neuroscience Inc, St. Petersburg, United States.
2- Department of Clinical Psychology, School of Behavioral Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
3- Department of Head, Brain, and Spinal Cord Injuries, Rofeideh Hospital, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
4- Applied Neuroscience Inc, St. Petersburg, United States.
Full-Text [PDF 1015 kb]
(851 Downloads)
| Abstract (HTML) (1987 Views)
Full-Text: (794 Views)
Introduction
Aphasia is a language disorder caused by traumatic or stroke-related brain damage. It frequently involves a variety of deteriorations and has a substantial effect on communication abilities and quality of life. Aphasia has been associated with a variety of structural and functional abnormalities. It has been associated with grey matter damage [1], white matter loss [2], and decreased brain connectivity [3]. Additionally, electrical activity is disrupted following brain damage. Changes in the membrane potential induced by energy deprivation result in an electrical impairment of the neurons, which leads to electroencephalography (EEG) changes. This energy deprivation is caused by a reduction of cerebral blood flow and can result in irreversible neural damage if the blood flow is not restored promptly [4]. According to Rabiller et al. [4], aphasia is characterized by an increase in slower frequency oscillations and a decrease in faster ones. Multiple studies have demonstrated that slow wave activity indicates pathological brain abnormality resulting from neurological damage [5]. Similarly, a significant relationship was reported between the location of the slow-wave activity and the lesion [6]. In addition, increased delta and theta activity in damaged linguistic regions of the left hemisphere has been demonstrated in patients with various aphasic diagnoses [7, 8]. During the phonological task, Spironelli et al. [9], found that aphasic patients exhibited lower levels of high beta activity in the left cluster of electrodes, which corresponded to the core of the damaged area. Moreover, higher levels of slow waves were significantly correlated with poor clinical outcomes in aphasia [7]. On the other hand, researchers also examined brain activity after recovery and linked it to a decrease in slow waves and an increase in fast waves in the aphasia brain [8, 9].
Due to the prevalence of aphasia and its complications, aphasia rehabilitation is essential and may improve the lives of aphasic patients. The recovery mechanisms depend on the severity of linguistic deficits, the size and location of the lesion, and the performance of residual unimpaired linguistic centers [10, 11].
Neuroplasticity is the key to restoring human functionality, as it ensures the brain’s capacity to adapt, change, self-repair, learn, and store memories [12]. For many years, neuromodulation, represented by neurofeedback (NFB), has been known as a potential therapeutic modality. It uses EEG displays in real-time to illustrate brain activity and enables self-regulation of brain activity by reducing excessive fast or slow waves, which are frequently observed in several disorders. Multiple disorders, including attention-deficit/hyperactivity disorder (ADHD) [13], depression [14], schizophrenia [15], reading disorders [16], and traumatic brain injury [17] have been reported to be effectively treated by NFB. In previous case studies, different NFB protocols have been utilized for the rehabilitation of aphasia. Rozelle and Budzynski [18] used beta1/theta NFB training on a male stroke patient to increase beta1 and decrease theta [18].They also reported improvements in speech fluency, word-finding, balance, coordination, attention, and concentration after NFB. Mroczkowska et al. [12], performed sensory motor rhythms (SMR)/theta NFT on a female stroke patient with aphasia to increase SMR and reduce theta. Positive effects on concentration, visual perception, and aphasia symptoms were reported by the authors. Nan et al. [19] suggested that alpha NFT could provide a variety of benefits for aphasia patients.
Relatively recently, low-resolution electromagnetic tomography analysis (LORETA) z-score NFB (LZNFB) was introduced to the market (Applied Neurosciences, Inc., USA). The application of a larger number of electrodes (i.e. scalp sensors) during treatment has the potential to expedite the effectiveness of this system [20]. The greatest advantage of LZNFB is its capacity to target brain network hubs known as Brodmann areas (BAs). In addition, it is capable of receiving instantaneous comparisons using a reference database of a healthy individual’s z-score. These immediate benefits facilitate the correlation between a patient’s symptoms and BAs [21]. This technology has recently been demonstrated to be an effective treatment for a variety of neuropsychiatric disorders, such as depression/anxiety and cognitive dysfunction [22], epilepsy [23], dementia [24], traumatic brain injury (TBI) [22], addiction [25], pain [26], and posttraumatic stress disorder (PTSD) [27]. Our recent case report demonstrated the potential of LZNFB in language rehabilitation for a patient with TBI [28]. To our knowledge, however, no study has demonstrated the effectiveness of this method on a group of aphasia patients. It was hypothesized that LZNFB would enhance language, working memory, and attention by increasing fast brain waves and decreasing slow ones. In this study, LZNFB was administered to aphasic patients. The QEEG/LORETA and behavioral tests were administered at baseline and after 15 LZNFB treatment sessions, and their results were analyzed.
Materials and methods
Participants
The study group consisted of 13 aphasic patients (five females and eight males) with Mean±SD ages of 46.53±12.95 years and Mean±SD education histories of 10.38±1.16 years, who had suffered a stroke or trauma (Table 1).

They were chosen based on the following criteria: 1) They were diagnosed as non-fluent aphasic patients during the acute phase (Table 1) at the time of the study, all patients had to be in a chronic state, as evidenced by an average of 27.84±5.55 months since the lesion (range: 7-60 months). Before the experimental session, residual language deficits in aphasic patients were assessed using the Persian version of the aphasia battery [29]. According to the aphasia assessment guide, A greater aphasia score indicates less severe symptoms. (0-25: Very severe; 26-50: Severe; 51-75: Moderate; 56-93: Mild).
Intervention
In 5-minute segments of eyes-closed resting states, power spectral analysis was carried out. The EEG was recorded from 19 scalp locations using a Medicom amplifier (Medicom MTD., Russia) and the Encephalan software. The QEEG data were edited and digitally analyzed using NeuroGuide software, version 3.0.9 and its comparative database. The protocol included language network-based LZNFB. The BA language network consisted of numbers 22, 39, 40, 41, 42, 44, and 45. In addition, in NFB, learning reinforcement was provided through the use of television shows or animations that grew in size when the defined difficulty thresholds were met.
LORETA source analysis was conducted using LORETA-KEY software, version 3.0.9 which employs a realistic head model [30]. Before and after LZNFB, the available neurocognitive testing batteries; Persian aphasia battery [31], forward and backward digit/word/non-word span [32], and Stroop test [33] were administered to evaluate the language, working memory, and attention.
Persian aphasia battery includes several sub-tests including naming, repeating, speed, utterance, auditory perception, utterance, and lexicon. For evaluating working memory performance, at the first step, two materials (digit, word, non-word) are presented to the patients and if they can repeat them correctly, several items are added accordingly. To estimate attention, we used computerized Stroop color and word tests, which involve congruent (word congruent with color) and incongruent items.
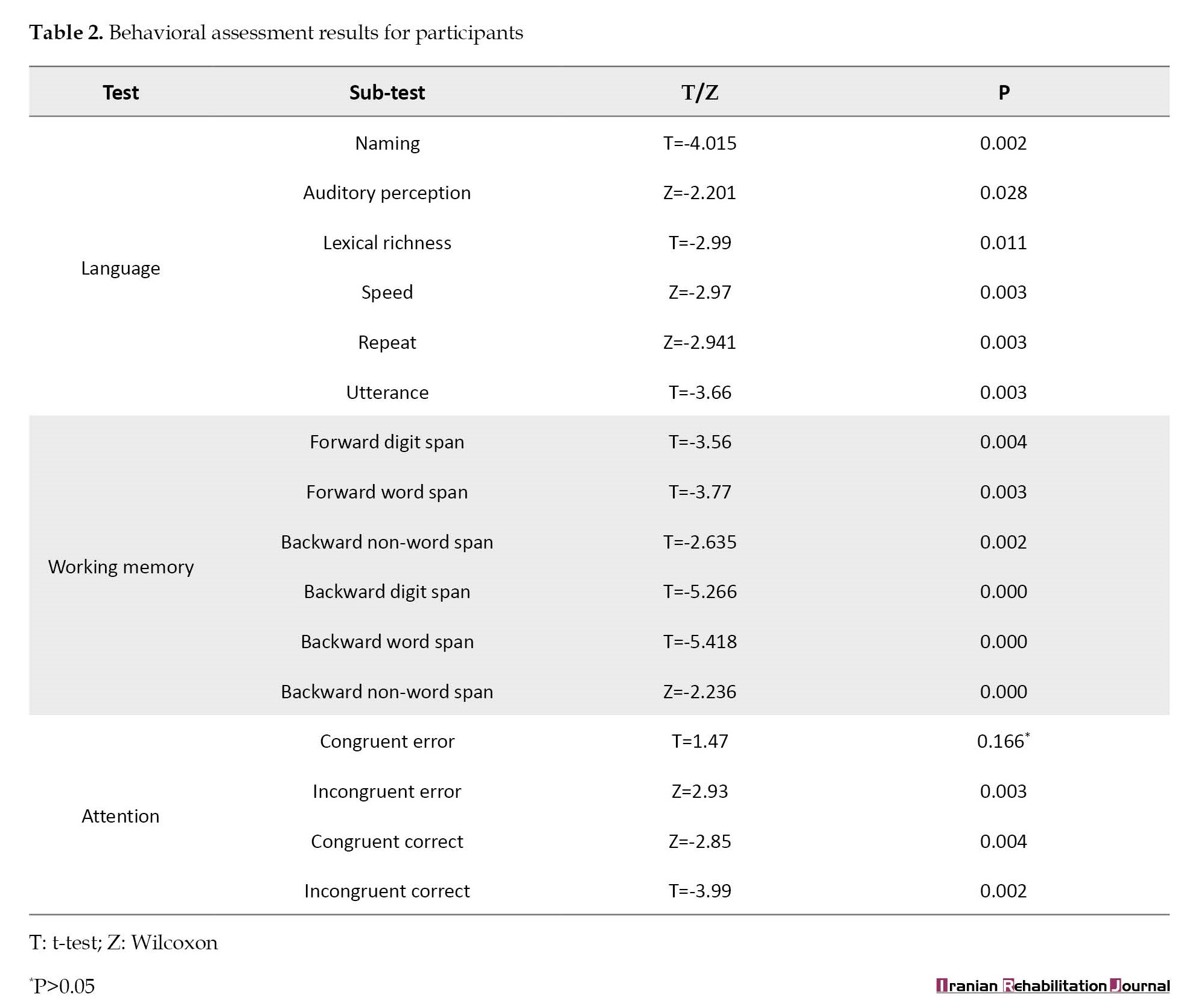
Then, the difference between baseline and post-treatment scores (1-2) was compared using parametric (t-test) or non-parametric (Wilcoxon) tests based on the symmetric/non-symmetric distribution of data using the SPSS software (Table 2).
Results
We investigated the effect of language network-based LZNFB on aphasia patients. The two components of our analysis were LORETA analysis and behavioral analysis. Figure 1 displays a summary of the LORETA analysis outcomes (in which the red and blue colors show increased and decreased neural activity, respectively). Analyses of behavior are shown in Table 2.
Figure 1 depicts the influence of LZNFB on the activity of high beta, beta, alpha, theta, and delta frequencies in a group of aphasic patients. The images depict coronal, sagittal, and axial views of the brain, from left to right. Red indicates regions of increased activity, while blue indicates regions of decreased activity in images. In addition, the maximum and minimum voxel estimated by LORETA are shown to the right of each image. In specific brain regions, the results indicated an increase in fast brain waves (high beta, beta, alpha) and a decrease in slow brain waves (theta, delta). At BA 11 in the orbital frontal gyrus (X=11, Y=41, Z=-26), the maximum increase in the current density at the high beta frequency was observed. In addition, the beta frequency showed the greatest increase in current density at BA 10 in the medial frontal gyrus (X=9, Y=66, Z=8), and the alpha frequency showed the greatest increase at BA 10 in the superior frontal gyrus (X=-17, Y=66, Z=-6). Furthermore, BA 29 in the posterior cingulate gyrus exhibited the greatest decline in the theta frequency current density (X=1, Y=-46, Z=22), while BA 24 in the cingulate gyrus exhibited the greatest decline in delta frequency current density (X=11, Y=17, Z=36).
Changes in the activity of high beta (a), beta (b), alpha (c), theta (d), delta, and (e) frequencies by LZNFB treatment. The images (from left to right) are coronal, sagittal, and axial views of the brain. The red color in images indicates regions of increased activity, and blue indicates regions of decreased activity
The behavioral analysis included the Persian version of the aphasia battery, the forward and backward digit/word/non-word span, and the Stroop test, which were acquired at baseline and the final LZNFB session. Each exam contained multiple questions. For each sub-test, the Shapiro-Wilk test was used to examine the normality of the data. For normal and non-normal distributions, the paired t-test (T) or Wilcoxon (Z) was subsequently used. *indicates significant changes (P<0.05) in Table 2. On the language test, participants’ naming, auditory perception, lexical richness, speed, repeat, and utterance items improved. In terms of working memory, forward and backward digit/word/non-word span scores increased. In addition, the attention test analysis revealed a higher score for congruent and incongruent correct answers and a reduction in incongruent error over LZNFB. However, despite a reduction in congruent error, the difference was not statistically significant (P>0.05).
Discussion
In this study, 13 non-fluent aphasic patients were treated with LZNFB (based on language network protocol) to improve their language, working memory, and attention. In a previous case study, we demonstrated that this method improves aphasic patient’s linguistic performance. However, there is almost no evidence in the scientific literature that LZNFB is effective for aphasia patients. In this study, patients were trained for 4-6 weeks using LORETA z-score neurofeedback based on the language network (2-3 sessions per week and 40 minutes per session). The QEEG/LORETA and behavioral tests were administered at baseline and during the final LZNFB session. Moreover, LORETA analysis was conducted using the NeuroGuide software, VERSION 3.0.9 and behavioral analysis was conducted employing the appropriate parametric and non-parametric tests. The LORETA analysis revealed an increase in fast brain waves and a decrease in slow brain waves in particular brain regions due to LZNFB. In addition, the behavioral analysis showed improvements in language, working memory, and attention, indicating that the LZNFB training altered the underlying physiology of the patients, which is discussed in the next section.
LORETA analysis
The LORETA analysis revealed elevated levels of high beta, beta, and alpha in the inferior frontal gyrus, medial frontal, and superior frontal regions, as well as decreased levels of theta and delta in the posterior cingulate and cingulate gyrus. Spironelli et al. [9] found that, compared with controls, aphasic patients exhibited lower levels of high beta activity in the left hemisphere, which corroborated the increased high beta observed in our study following treatment. Therefore, an increase in high beta in the inferior frontal following treatment may be indicative of a reduction in aphasia symptoms. The inferior frontal gyrus (the classical Broca area) has traditionally been regarded frontal lobe’s primary language hub. According to Hazem et al., this region is associated with several language functions [34]. Our findings also showed an increase in beta activity in the medial frontal region. The beta frequency band is a recognized indicator of cognitive arousal and language processing and it is typically associated with active wakefulness and alertness [35, 36]. The medial frontal cortex, as demonstrated by increased beta in our study, is involved in higher-order cognition, especially episodic memory, and has a strong connection to the hippocampus, cingulate, entorhinal cortices, and anterior thalamus [37].
Previous case studies on stroke rehabilitation have reported an increase in high beta, beta, and alpha, along with a decrease in theta over NFB [12, 18, 19]. Earlier findings associating recovery with decreased slow waves and increased fast waves in the aphasia brain also support our findings [8, 38]. Decreased delta in the cingulate can support the recovery efficacy of LZFB. The delta band identifies pathological brain abnormality caused by neuronal damage [10]. Therefore, a smaller delta in the cingulate gyrus may indicate a greater activation of this region relative to LZNFB. Our findings of decreased delta activity in the cingulate cortical region are in line with previous studies [39, 40]. Geranmayeh et al. [39], demonstrated that the cingulate was activated when aphasic patients attempted to speak. In addition, they discovered a correlation between activity in the cingulate gyrus and the degree of spontaneous speech production recovery after stroke. Similarly, Brownsett et al. [40] demonstrated a positive correlation between cingulate activity during a language task and performance on the picture description task. These data may provide insight into the neural substrate of aphasia, which can be modified using NFB [40].
Behavioral analysis
Language, working memory, and attention showed significant changes (P<0.05) as a result of LZNFB based on the language network training. Participants’ naming, auditory perception, lexical richness, speed, repeat, and utterance of items improved on the language test. Moreover, in terms of working memory, forward and backward digit/word/non-word span scores increased. In addition, analysis of attention tests revealed an increase in congruent and incongruent correct responses and a decrease in incongruent error over LZNFB.
Both LORETA and behavioral analyses demonstrated the beneficial effects of LZNFB on aphasia patients. Our findings are consistent with previous research demonstrating the efficiency of LZNFB on several disorders [22-27].
Notably, our LZNFB training was limited to language networks only. However, its impact was not limited to language proficiency. Rather, working memory and attention skills were also enhanced. These alterations may be attributed to the close relationship between language and cognitive skills, which has been noted in previous studies [41, 42].
Conclusion
LZNFB appeared to have induced neural changes in aphasic patients. These changes were associated with improved aphasia symptoms in terms of language, working memory, and attention. These results are very encouraging as they suggest that LZNFB training could provide a range of improvements and offer new hope to people with aphasia who could not obtain satisfying improvements through traditional therapies.
Limitations
Lack of a control group and heterogeneity in age, education, cause, and severity of aphasia, which could influence language function differently, were limitations of this study. In addition, given the small sample size, our study may lack the statistical power to detect a significant difference. However, these findings could be considered exploratory. Future research can examine the brain and behavioral changes in larger samples and possibly in a homogeneous group of aphasia patients.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them. A written consent has been obtained from the subjects. Principles of the Helsinki Convention was also observed.
Funding
This research did not receive any grant from funding agencies in the public, commercial or non-profit sectors
Authors' contributions
Conceptualization and supervision: Hayat Ameri, Masoud Nosratabadi and Seyed Majid Akhavan Hejazi; Data collection, data analysis, investigating and writing original draft: Farnaz Faridi; Review and edit: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to acknowledge the contributions and helpful suggestions provided by Applied Neuroscience Inc.
References
Aphasia is a language disorder caused by traumatic or stroke-related brain damage. It frequently involves a variety of deteriorations and has a substantial effect on communication abilities and quality of life. Aphasia has been associated with a variety of structural and functional abnormalities. It has been associated with grey matter damage [1], white matter loss [2], and decreased brain connectivity [3]. Additionally, electrical activity is disrupted following brain damage. Changes in the membrane potential induced by energy deprivation result in an electrical impairment of the neurons, which leads to electroencephalography (EEG) changes. This energy deprivation is caused by a reduction of cerebral blood flow and can result in irreversible neural damage if the blood flow is not restored promptly [4]. According to Rabiller et al. [4], aphasia is characterized by an increase in slower frequency oscillations and a decrease in faster ones. Multiple studies have demonstrated that slow wave activity indicates pathological brain abnormality resulting from neurological damage [5]. Similarly, a significant relationship was reported between the location of the slow-wave activity and the lesion [6]. In addition, increased delta and theta activity in damaged linguistic regions of the left hemisphere has been demonstrated in patients with various aphasic diagnoses [7, 8]. During the phonological task, Spironelli et al. [9], found that aphasic patients exhibited lower levels of high beta activity in the left cluster of electrodes, which corresponded to the core of the damaged area. Moreover, higher levels of slow waves were significantly correlated with poor clinical outcomes in aphasia [7]. On the other hand, researchers also examined brain activity after recovery and linked it to a decrease in slow waves and an increase in fast waves in the aphasia brain [8, 9].
Due to the prevalence of aphasia and its complications, aphasia rehabilitation is essential and may improve the lives of aphasic patients. The recovery mechanisms depend on the severity of linguistic deficits, the size and location of the lesion, and the performance of residual unimpaired linguistic centers [10, 11].
Neuroplasticity is the key to restoring human functionality, as it ensures the brain’s capacity to adapt, change, self-repair, learn, and store memories [12]. For many years, neuromodulation, represented by neurofeedback (NFB), has been known as a potential therapeutic modality. It uses EEG displays in real-time to illustrate brain activity and enables self-regulation of brain activity by reducing excessive fast or slow waves, which are frequently observed in several disorders. Multiple disorders, including attention-deficit/hyperactivity disorder (ADHD) [13], depression [14], schizophrenia [15], reading disorders [16], and traumatic brain injury [17] have been reported to be effectively treated by NFB. In previous case studies, different NFB protocols have been utilized for the rehabilitation of aphasia. Rozelle and Budzynski [18] used beta1/theta NFB training on a male stroke patient to increase beta1 and decrease theta [18].They also reported improvements in speech fluency, word-finding, balance, coordination, attention, and concentration after NFB. Mroczkowska et al. [12], performed sensory motor rhythms (SMR)/theta NFT on a female stroke patient with aphasia to increase SMR and reduce theta. Positive effects on concentration, visual perception, and aphasia symptoms were reported by the authors. Nan et al. [19] suggested that alpha NFT could provide a variety of benefits for aphasia patients.
Relatively recently, low-resolution electromagnetic tomography analysis (LORETA) z-score NFB (LZNFB) was introduced to the market (Applied Neurosciences, Inc., USA). The application of a larger number of electrodes (i.e. scalp sensors) during treatment has the potential to expedite the effectiveness of this system [20]. The greatest advantage of LZNFB is its capacity to target brain network hubs known as Brodmann areas (BAs). In addition, it is capable of receiving instantaneous comparisons using a reference database of a healthy individual’s z-score. These immediate benefits facilitate the correlation between a patient’s symptoms and BAs [21]. This technology has recently been demonstrated to be an effective treatment for a variety of neuropsychiatric disorders, such as depression/anxiety and cognitive dysfunction [22], epilepsy [23], dementia [24], traumatic brain injury (TBI) [22], addiction [25], pain [26], and posttraumatic stress disorder (PTSD) [27]. Our recent case report demonstrated the potential of LZNFB in language rehabilitation for a patient with TBI [28]. To our knowledge, however, no study has demonstrated the effectiveness of this method on a group of aphasia patients. It was hypothesized that LZNFB would enhance language, working memory, and attention by increasing fast brain waves and decreasing slow ones. In this study, LZNFB was administered to aphasic patients. The QEEG/LORETA and behavioral tests were administered at baseline and after 15 LZNFB treatment sessions, and their results were analyzed.
Materials and methods
Participants
The study group consisted of 13 aphasic patients (five females and eight males) with Mean±SD ages of 46.53±12.95 years and Mean±SD education histories of 10.38±1.16 years, who had suffered a stroke or trauma (Table 1).

They were chosen based on the following criteria: 1) They were diagnosed as non-fluent aphasic patients during the acute phase (Table 1) at the time of the study, all patients had to be in a chronic state, as evidenced by an average of 27.84±5.55 months since the lesion (range: 7-60 months). Before the experimental session, residual language deficits in aphasic patients were assessed using the Persian version of the aphasia battery [29]. According to the aphasia assessment guide, A greater aphasia score indicates less severe symptoms. (0-25: Very severe; 26-50: Severe; 51-75: Moderate; 56-93: Mild).
Intervention
In 5-minute segments of eyes-closed resting states, power spectral analysis was carried out. The EEG was recorded from 19 scalp locations using a Medicom amplifier (Medicom MTD., Russia) and the Encephalan software. The QEEG data were edited and digitally analyzed using NeuroGuide software, version 3.0.9 and its comparative database. The protocol included language network-based LZNFB. The BA language network consisted of numbers 22, 39, 40, 41, 42, 44, and 45. In addition, in NFB, learning reinforcement was provided through the use of television shows or animations that grew in size when the defined difficulty thresholds were met.
LORETA source analysis was conducted using LORETA-KEY software, version 3.0.9 which employs a realistic head model [30]. Before and after LZNFB, the available neurocognitive testing batteries; Persian aphasia battery [31], forward and backward digit/word/non-word span [32], and Stroop test [33] were administered to evaluate the language, working memory, and attention.
Persian aphasia battery includes several sub-tests including naming, repeating, speed, utterance, auditory perception, utterance, and lexicon. For evaluating working memory performance, at the first step, two materials (digit, word, non-word) are presented to the patients and if they can repeat them correctly, several items are added accordingly. To estimate attention, we used computerized Stroop color and word tests, which involve congruent (word congruent with color) and incongruent items.
Then, the difference between baseline and post-treatment scores (1-2) was compared using parametric (t-test) or non-parametric (Wilcoxon) tests based on the symmetric/non-symmetric distribution of data using the SPSS software (Table 2).

Results
We investigated the effect of language network-based LZNFB on aphasia patients. The two components of our analysis were LORETA analysis and behavioral analysis. Figure 1 displays a summary of the LORETA analysis outcomes (in which the red and blue colors show increased and decreased neural activity, respectively). Analyses of behavior are shown in Table 2.
Figure 1 depicts the influence of LZNFB on the activity of high beta, beta, alpha, theta, and delta frequencies in a group of aphasic patients. The images depict coronal, sagittal, and axial views of the brain, from left to right. Red indicates regions of increased activity, while blue indicates regions of decreased activity in images. In addition, the maximum and minimum voxel estimated by LORETA are shown to the right of each image. In specific brain regions, the results indicated an increase in fast brain waves (high beta, beta, alpha) and a decrease in slow brain waves (theta, delta). At BA 11 in the orbital frontal gyrus (X=11, Y=41, Z=-26), the maximum increase in the current density at the high beta frequency was observed. In addition, the beta frequency showed the greatest increase in current density at BA 10 in the medial frontal gyrus (X=9, Y=66, Z=8), and the alpha frequency showed the greatest increase at BA 10 in the superior frontal gyrus (X=-17, Y=66, Z=-6). Furthermore, BA 29 in the posterior cingulate gyrus exhibited the greatest decline in the theta frequency current density (X=1, Y=-46, Z=22), while BA 24 in the cingulate gyrus exhibited the greatest decline in delta frequency current density (X=11, Y=17, Z=36).
Changes in the activity of high beta (a), beta (b), alpha (c), theta (d), delta, and (e) frequencies by LZNFB treatment. The images (from left to right) are coronal, sagittal, and axial views of the brain. The red color in images indicates regions of increased activity, and blue indicates regions of decreased activity
The behavioral analysis included the Persian version of the aphasia battery, the forward and backward digit/word/non-word span, and the Stroop test, which were acquired at baseline and the final LZNFB session. Each exam contained multiple questions. For each sub-test, the Shapiro-Wilk test was used to examine the normality of the data. For normal and non-normal distributions, the paired t-test (T) or Wilcoxon (Z) was subsequently used. *indicates significant changes (P<0.05) in Table 2. On the language test, participants’ naming, auditory perception, lexical richness, speed, repeat, and utterance items improved. In terms of working memory, forward and backward digit/word/non-word span scores increased. In addition, the attention test analysis revealed a higher score for congruent and incongruent correct answers and a reduction in incongruent error over LZNFB. However, despite a reduction in congruent error, the difference was not statistically significant (P>0.05).
Discussion
In this study, 13 non-fluent aphasic patients were treated with LZNFB (based on language network protocol) to improve their language, working memory, and attention. In a previous case study, we demonstrated that this method improves aphasic patient’s linguistic performance. However, there is almost no evidence in the scientific literature that LZNFB is effective for aphasia patients. In this study, patients were trained for 4-6 weeks using LORETA z-score neurofeedback based on the language network (2-3 sessions per week and 40 minutes per session). The QEEG/LORETA and behavioral tests were administered at baseline and during the final LZNFB session. Moreover, LORETA analysis was conducted using the NeuroGuide software, VERSION 3.0.9 and behavioral analysis was conducted employing the appropriate parametric and non-parametric tests. The LORETA analysis revealed an increase in fast brain waves and a decrease in slow brain waves in particular brain regions due to LZNFB. In addition, the behavioral analysis showed improvements in language, working memory, and attention, indicating that the LZNFB training altered the underlying physiology of the patients, which is discussed in the next section.
LORETA analysis
The LORETA analysis revealed elevated levels of high beta, beta, and alpha in the inferior frontal gyrus, medial frontal, and superior frontal regions, as well as decreased levels of theta and delta in the posterior cingulate and cingulate gyrus. Spironelli et al. [9] found that, compared with controls, aphasic patients exhibited lower levels of high beta activity in the left hemisphere, which corroborated the increased high beta observed in our study following treatment. Therefore, an increase in high beta in the inferior frontal following treatment may be indicative of a reduction in aphasia symptoms. The inferior frontal gyrus (the classical Broca area) has traditionally been regarded frontal lobe’s primary language hub. According to Hazem et al., this region is associated with several language functions [34]. Our findings also showed an increase in beta activity in the medial frontal region. The beta frequency band is a recognized indicator of cognitive arousal and language processing and it is typically associated with active wakefulness and alertness [35, 36]. The medial frontal cortex, as demonstrated by increased beta in our study, is involved in higher-order cognition, especially episodic memory, and has a strong connection to the hippocampus, cingulate, entorhinal cortices, and anterior thalamus [37].
Previous case studies on stroke rehabilitation have reported an increase in high beta, beta, and alpha, along with a decrease in theta over NFB [12, 18, 19]. Earlier findings associating recovery with decreased slow waves and increased fast waves in the aphasia brain also support our findings [8, 38]. Decreased delta in the cingulate can support the recovery efficacy of LZFB. The delta band identifies pathological brain abnormality caused by neuronal damage [10]. Therefore, a smaller delta in the cingulate gyrus may indicate a greater activation of this region relative to LZNFB. Our findings of decreased delta activity in the cingulate cortical region are in line with previous studies [39, 40]. Geranmayeh et al. [39], demonstrated that the cingulate was activated when aphasic patients attempted to speak. In addition, they discovered a correlation between activity in the cingulate gyrus and the degree of spontaneous speech production recovery after stroke. Similarly, Brownsett et al. [40] demonstrated a positive correlation between cingulate activity during a language task and performance on the picture description task. These data may provide insight into the neural substrate of aphasia, which can be modified using NFB [40].
Behavioral analysis
Language, working memory, and attention showed significant changes (P<0.05) as a result of LZNFB based on the language network training. Participants’ naming, auditory perception, lexical richness, speed, repeat, and utterance of items improved on the language test. Moreover, in terms of working memory, forward and backward digit/word/non-word span scores increased. In addition, analysis of attention tests revealed an increase in congruent and incongruent correct responses and a decrease in incongruent error over LZNFB.
Both LORETA and behavioral analyses demonstrated the beneficial effects of LZNFB on aphasia patients. Our findings are consistent with previous research demonstrating the efficiency of LZNFB on several disorders [22-27].
Notably, our LZNFB training was limited to language networks only. However, its impact was not limited to language proficiency. Rather, working memory and attention skills were also enhanced. These alterations may be attributed to the close relationship between language and cognitive skills, which has been noted in previous studies [41, 42].
Conclusion
LZNFB appeared to have induced neural changes in aphasic patients. These changes were associated with improved aphasia symptoms in terms of language, working memory, and attention. These results are very encouraging as they suggest that LZNFB training could provide a range of improvements and offer new hope to people with aphasia who could not obtain satisfying improvements through traditional therapies.
Limitations
Lack of a control group and heterogeneity in age, education, cause, and severity of aphasia, which could influence language function differently, were limitations of this study. In addition, given the small sample size, our study may lack the statistical power to detect a significant difference. However, these findings could be considered exploratory. Future research can examine the brain and behavioral changes in larger samples and possibly in a homogeneous group of aphasia patients.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed of the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information and were free to leave the study whenever they wished, and if desired, the research results would be available to them. A written consent has been obtained from the subjects. Principles of the Helsinki Convention was also observed.
Funding
This research did not receive any grant from funding agencies in the public, commercial or non-profit sectors
Authors' contributions
Conceptualization and supervision: Hayat Ameri, Masoud Nosratabadi and Seyed Majid Akhavan Hejazi; Data collection, data analysis, investigating and writing original draft: Farnaz Faridi; Review and edit: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to acknowledge the contributions and helpful suggestions provided by Applied Neuroscience Inc.
References
- Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, et al. Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics. 2009; 22(4):370-83. [DOI:10.1016/j.jneuroling.2008.12.001] [PMID]
- Hillis AE, Beh YY, Sebastian R, Breining B, Tippett DC, Wright A, et al. Predicting recovery in acute poststroke aphasia. Annals of Neurology. 2018; 83(3):612-22. [DOI:10.1002/ana.25184] [PMID]
- Beuter A, Balossier A, Vassal F, Hemm S, Volpert V. Cortical stimulation in aphasia following ischemic stroke: Toward model-guided electrical neuromodulation. Biological Cybernetics. 2020; 114(1):5-21. [DOI:10.1007/s00422-020-00818-w] [PMID]
- Rabiller G, He JW, Nishijima Y, Wong A, Liu J. Perturbation of brain oscillations after ischemic stroke: A potential biomarker for post-stroke function and therapy. International Journal of Molecular Sciences. 2015; 16(10):25605-40. [DOI:10.3390/ijms161025605] [PMID]
- de Jongh A, de Munck JC, Baayen JC, Jonkman EJ, Heethaar RM, van Dijk BW. The localization of spontaneous brain activity: First results in patients with cerebral tumors. Clinical Neurophysiology. 2001; 112(2):378-85. [DOI:10.1016/S1388-2457(00)00526-5] [PMID]
- Nagata K, Mizukami M, Araki G, Kawase T, Hirano M. Topographic electroencephalographic study of cerebral infarction using computed mapping of the EEG. Journal of Cerebral Blood Flow and Metabolism. 1982; 2(1):79-88. [DOI:10.1038/jcbfm.1982.9] [PMID]
- Szelies B, Mielke R, Kessler J, Heiss WD. Prognostic relevance of quantitative topographical EEG in patients with poststroke aphasia. Brain and language. 2002; 82(1):87-94. [DOI:10.1016/S0093-934X(02)00004-4] [PMID]
- de Jongh A, Baayen JC, de Munck JC, Heethaar RM, Vandertop WP, Stam CJ. The influence of brain tumor treatment on pathological delta activity in MEG. Neuroimage. 2003; 20(4):2291-301. [DOI:10.1016/j.neuroimage.2003.07.030] [PMID]
- Spironelli C, Manfredi M, Angrilli A. Beta EEG band: A measure of functional brain damage and language reorganization in aphasic patients after recovery. Cortex. 2013; 49(10):2650-60. [DOI:10.1016/j.cortex.2013.05.003] [PMID]
- Spironelli C, Angrilli A. EEG delta band as a marker of brain damage in aphasic patients after recovery of language. Neuropsychologia. 2009; 47(4):988-94. [DOI:10.1016/j.neuropsychologia.2008.10.019] [PMID]
- Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. Current Opinion in Neurology. 2005; 18(4):429-34. [DOI:10.1097/01.wco.0000168081.76859.c1] [PMID]
- Mroczkowska D, Białkowska J, Rakowska A. Neurofeedback as supportive therapy after stroke. Case report. Postępy Psychiatrii i Neurologii. 2014; 23(4):190-201. [DOI:10.1016/j.pin.2014.09.002]
- Enriquez-Geppert S, Smit D, Pimenta MG, Arns M. Neurofeedback as a treatment intervention in ADHD: Current evidence and practice. Current Psychiatry Reports. 2019; 21(6):46. [DOI:10.1007/s11920-019-1021-4] [PMID]
- Grin-Yatsenko VA, Othmer S, Ponomarev VA, Evdokimov SA, Konoplev YY, Kropotov JD. Infra-low frequency neurofeedback in depression: Three case studies. NeuroRegulation. 2018; 5(1):30-42. [DOI:10.15540/nr.5.1.30]
- Hirano Y, Tamura S. Recent findings on neurofeedback training for auditory hallucinations in schizophrenia. Current Opinion in Psychiatry. 2021; 34(3):245-52. [DOI:10.1097/YCO.0000000000000693] [PMID]
- Nazari M, Karkoodi K, Alizadeh A. Performance and physiological responses of milk-fed calves to coated calcium butyrate supplementation. South African Journal of Animal Science. 2012; 42(3):296-303. [DOI:10.4314/sajas.v42i3.12]
- Thornton KE, Carmody DP. Electroencephalogram biofeedback for reading disability and traumatic brain injury. Child and Adolescent Psychiatric Clinics of North America. 2005; 14(1):137-62. [DOI:10.1016/j.chc.2004.07.001] [PMID]
- Rozelle GR, Budzynski TH. Neurotherapy for stroke rehabilitation: A single case study. Biofeedback and Self-Regulation. 1995; 20(3):211-28. [DOI:10.1007/BF01474514] [PMID]
- Nan W, Dias APB, Rosa AC. Neurofeedback training for cognitive and motor function rehabilitation in chronic stroke: Two case reports. Frontiers in Neurology. 2019; 10:800.[DOI:10.3389/fneur.2019.00800] [PMID]
- Koberda JL, Moses A, Koberda L, Koberda P. Cognitive enhancement using 19-electrode z-score neurofeedback. Journal of Neurotherapy. 2012; 16(3):224-30. [DOI:10.1080/10874208.2012.705769]
- Thatcher RW. Validity and reliability of quantitative electroencephalography. Journal of Neurotherapy. 2010; 14(2):122-52. [DOI:10.1080/10874201003773500]
- Koberda J. LORETA Z-score neurofeedback-effectiveness in rehabilitation of patients suffering from traumatic brain injury. Journal of Neurology and Neurobiology. 2015; 1(4):1-9. [DOI:10.16966/2379-7150.113]
- Frey LC, Koberda J. LORETA Z-score neurofeedback in patients with medically refractory epilepsy. Journal of Neurology and Neurobiology. 2015; 1:1-4. [DOI:10.16966/2379-7150.102]
- Lucas Koberda J. Z-score LORETA neurofeedback as a potential therapy in cognitive dysfunction and dementia. Journal of Psychology & Clinical Psychiatry. 2014; 1(6):00037. [DOI:10.15406/jpcpy.2014.01.00037]
- Faridi A, Taremian F, Thatcher RW, Dadashi M, Moloodi R. Comparing LORETA Z score neurofeedback and cognitive rehabilitation regarding their effectiveness in reducing craving in opioid addicts. Basic and Clinical Neuroscience. 2022; 13(1):81-96. [DOI:10.32598/bcn.2021.1946.1] [PMID]
- Prinsloo S, Rosenthal DI, Lyle R, Garcia SM, Gabel-Zepeda S, Cannon R, et al. Exploratory study of low resolution electromagnetic tomography (LORETA) real-time z-score feedback in the treatment of pain in patients with head and neck cancer. Brain Topography. 2019; 32(2):283-5. [DOI:10.1007/s10548-018-0686-z] [PMID]
- Bell AN. Tuning the traumatized brain, mind, and heart: Loreta z-score neurofeedback and HRV biofeedback for chronic PTSD [PhD dissertation]. Oakland: Saybrook University; 2018. [Link]
- Faridi F, Ameri H, Nosratabadi M, Hejazi SMA, Thatcher R. Language rehabilitation of TBI patient by Loreta Z score Neurofeedback. NeuroRegulation. 2021; 8(2):121-6. [DOI:10.15540/nr.8.2.121]
- Nilipour R, Pour Shahbaz A, Ghoreishi ZS, Yousefi A. [Reliability and validity of Persian aphasia battery test (Persian)]. Iranian Journal of Ageing. 2016; 10(4):182-91. [Link]
- Wagner M, Fuchs M, Kastner J. Evaluation of sLORETA in the presence of noise and multiple sources. Brain Topography. 2004; 16(4):277-80. [DOI:10.1023/B:BRAT.0000032865.58382.62] [PMID]
- Nilipour R, Pourshahbaz A, Ghoreyshi ZS. Reliability and validity of bedside version of Persian WAB (P-WAB-1). Basic and Clinical Neuroscience. 2014; 5(4):253-8. [PMID] [PMCID]
- Hilbert S, Nakagawa TT, Puci P, Zech A, Bühner M. The digit span backward task. European Journal of Psychological Assessment. 2014; 31(3):174-80. [DOI:10.1027/1015-5759/a000223]
- Jensen AR. Scoring the stroop test. Acta Psychologica. 1965; 24(5):398-408. [DOI:10.1016/0001-6918(65)90024-7] [PMID]
- Hazem SR, Awan M, Lavrador JP, Patel S, Wren HM, Lucena O, et al. Middle Frontal Gyrus and Area 55b: Perioperative Mapping and Language Outcomes. Frontiers in Neurology. 2021; 12:194. [DOI:10.3389/fneur.2021.646075] [PMID]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000; 101(2):243-76.[DOI:10.1016/S0306-4522(00)00353-5] [PMID]
- Spironelli C, Angrilli A. Developmental aspects of language lateralization in delta, theta, alpha and beta EEG bands. Biological Psychology. 2010; 85(2):258-67. [DOI:10.1016/j.biopsycho.2010.07.011] [PMID]
- Nestor PG, Nakamura M, Niznikiewicz M, Thompson E, Levitt JJ, Choate V, et al. In search of the functional neuroanatomy of sociality: MRI subdivisions of orbital frontal cortex and social cognition. Social Cognitive and Affective Neuroscience. 2013; 8(4):460-7. [DOI:10.1093/scan/nss018] [PMID]
- Spironelli C, Bergamaschi S, Mondini S, Villani D, Angrilli A. Functional plasticity in Alzheimer's disease: Effect of cognitive training on language-related ERP components. Neuropsychologia. 2013; 51(8):1638-48. [DOI:10.1016/j.neuropsychologia.2013.05.007] [PMID]
- Geranmayeh F, Chau TW, Wise RJS, Leech R, Hampshire A. Domain-general subregions of the medial prefrontal cortex contribute to recovery of language after stroke. Brain. 2017; 140(7):1947-58. [DOI:10.1093/brain/awx134] [PMID]
- Brownsett SL, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJ. Cognitive control and its impact on recovery from aphasic stroke. Brain. 2014; 137(Pt 1):242-54. [DOI:10.1093/brain/awt289] [PMID]
- Harris CL. Language and cognition. In: Nadel L, editor. Encyclopedia of cognitive science. Toronto: John Wiley & Sons, Ltd; 2006. [DOI:10.1002/0470018860.s00559]
- Perlovsky L, Sakai KL. Language and cognition. Frontiers in Behavioral Neuroscience. 2014; 8:436. [DOI:10.3389/fnbeh.2014.00436] [PMID]
Article type: Original Research Articles |
Subject:
Neurorehabilitation
Received: 2022/10/7 | Accepted: 2023/08/19 | Published: 2023/12/1
Received: 2022/10/7 | Accepted: 2023/08/19 | Published: 2023/12/1
Send email to the article author






