Volume 22, Issue 1 (March 2024)
Iranian Rehabilitation Journal 2024, 22(1): 95-106 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Midha D, Arumugam N. Therapeutic Application of Multichannel Transcranial Direct Stimulation on Cognitive Domains and Motor Functions of Paretic Hand. Iranian Rehabilitation Journal 2024; 22 (1) :95-106
URL: http://irj.uswr.ac.ir/article-1-1845-en.html
URL: http://irj.uswr.ac.ir/article-1-1845-en.html
1- Department of Physiotherapy, Punjabi University, Patiala, India.
Keywords: Stroke, Rehabilitation, Transcranial direct currentstimulation, Hemiplegia, Non-invasive brainstimulation
Full-Text [PDF 739 kb]
(782 Downloads)
| Abstract (HTML) (3053 Views)
Full-Text: (511 Views)
Introduction
Stroke has emerged as a major public health concern in the past decade, and its prevalence may continue to rise, especially in developing countries due to shifts in demographic profiles. Hence, stroke occupies a prominent position on the agenda of various health-related issues facing the general public in the 21st century. It represents an important area for general health research, necessitating robust interventions aimed at achieving high survival rates and enhancing the quality of life for stroke survivors [1]. Among the varied symptoms, motor impairment ranks as one of the most prevalent deficits in stroke survivors (PABLO) [2], making it a major contributor to physical and mental disability among them. Motor recovery following stroke is one of the key concerns of rehabilitation professionals.
To achieve optimal recovery and develop effective intervention strategies for compensating for brain injury, it is essential to harness the intrinsic capacity of viable cortical networks. However, despite the implementation of novel therapeutic approaches, the effect size of individual interventions may sometimes be insufficient to induce complete recovery [3].
The human brain is a complex network of multiple cortical networks. Brain stimulation modulates network activity. However, targeting a single area of the brain may not yield satisfactory results [4]. The restoration of motor functions relies on the viability of various circuits connecting the hemispheres, with motor networks closely interacting with nearby cognitive networks. Hence, the influence of cognitive networks on motor recovery is significant and cannot be overlooked. Motor learning exhibits a strong association with various cognitive domains. The same principle can be applied by targeting more than one corresponding neuronal circuit in the cerebral cortex via neuro-modulation [5].
Neuro-modulation is achieved by applying a weak electric current, which causes the activation or deactivation of excitable tissue, leading to improved patient outcomes. Transcranial direct current stimulation (tDCS) is one of the key neuromodulation tools with therapeutic benefits in post-stroke rehabilitation. Many authors have successfully used tDCS in their studies. However, in most of the trials, the authors provided tDCS over a single cortical region at different time periods to get the desired outcome [6]. Some authors have also stated that tDCS may have better results with a better understanding of neural networks and their connections with adjacent regions along with conventional rehabilitation. Hence, the aim of the current trial was to investigate the effect of multichannel transcranial direct current stimulation (M-tDCS) on global recovery following stroke.
Materials and Methods
Participant screening and recruitment
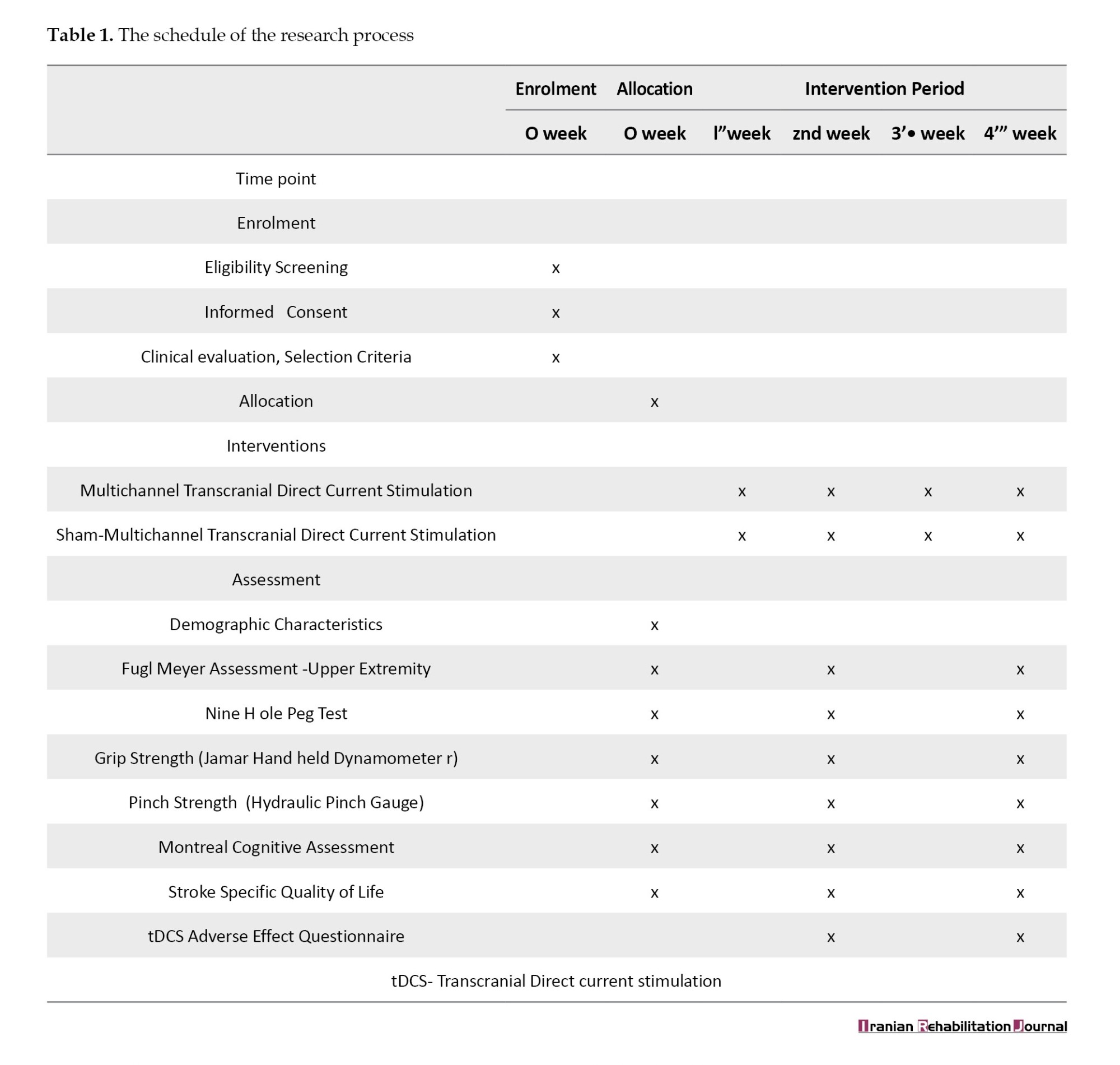
The present study was a prospective, parallel-group, double-blinded randomized controlled trial. Stroke survivors in the age range of 40-75 years were selected from neuro-physiotherapy rehabilitation units/centers in Patiala City of Punjab, India from January 2020 to December 2021. Thirty-five individuals were screened, of whom 14 individuals fulfilled the eligibility criteria of the study. The participation schedule was prepared according to the guidelines of intervention trials (Table 1) [7].
Eligibility Criteria
Inclusion criteria
Participants were selected as per the following criteria: Both male and female individuals, medically diagnosed with cortical stroke (ischemic), age between 40 and 75 years, preserved range of motion of the wrist (approximately 10 degrees), modified Ashworth scale grade <2 (in major muscle groups in the upper extremity and lower extremity), mini-mental state examination (MMSE) score between 18-23, and those with ambulatory care.
Exclusion criteria
Individuals who did not meet the inclusion criteria were excluded from the study. Exclusion criteria included:
Medical diagnosis of hemorrhagic stroke; history of neurological disorders other than stroke; musculoskeletal disorders affecting upper or lower extremity motor function; visual analog scale score exceeding 4 in the upper or lower extremity; individuals with psychosomatic illnesses; medically unstable individuals with a history of cardiovascular or respiratory illnesses; systemic illnesses; presence of metallic implants; pregnancy; uncontrolled hypertension; previous participation in any other pharmacological or rehabilitation study; sensory disorders; lack of interest in participating in the study.
Participant allocation
All subjects received participant information sheets in their local language and written consent was taken from them before the study. Complete information was given to participants about the objectives of the study, study procedure, and major/minor risks and/or benefits. Participants were also told that their enrolment was entirely voluntary and that they had the full right to withdraw from the study at any stage.
Randomization, allocation, and blinding
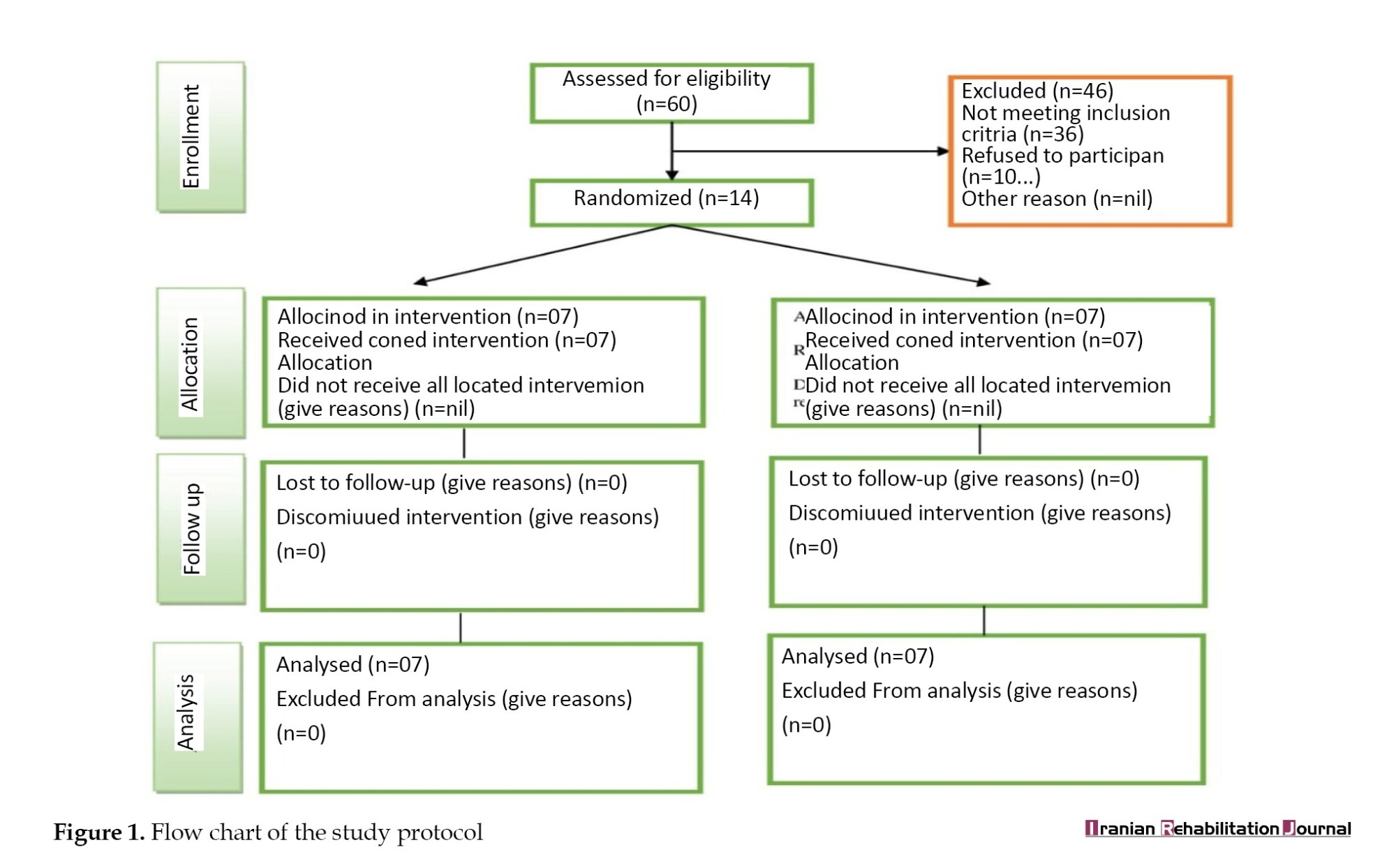
Out of 35 individuals, 14 participants were recruited for the trial. The investigator prepared a randomization schedule before the start of the study using a computer-generated random list of participants. Random allocation of participants was done, with 7 individuals in each group. The computer-generated list was sealed in an opaque envelope and given to a third person who had no direct or indirect involvement in the study. The participants and assessors were blinded to the intervention and allocation throughout the entire study process. The schematic consolidated standard of reporting trials flow chart for the study protocol is shown in Figure 1.
Enrolment and baseline measurements and clinical trial registration
The demographic profile of the participants included their names, age, and gender. Baseline data of all the participants were documented, which included their mechanism of stroke, stroke duration, hemiparetic side, participant handedness, MMSE score, and the National Institutes of Health Stroke Scale (NIHSS) score. The Standard protocol items: Recommendations for Interventional Trials (SPIRIT) statement was used for documenting the schedule of study participants. The enrolment process was initiated from January 2020 onwards.
Interventions
M-tDCS procedure
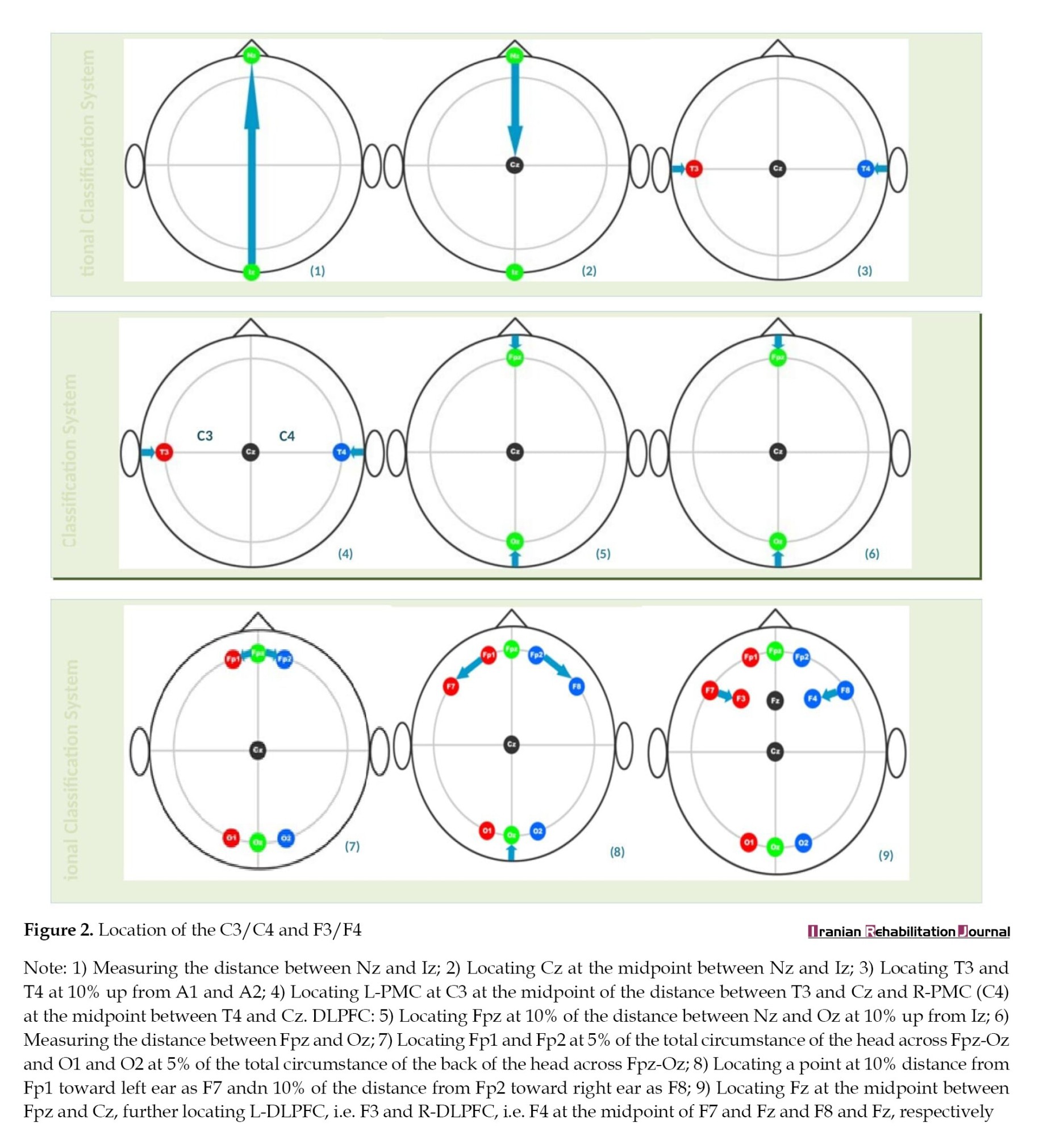
Participants in the experimental group were given M-tDCS on the underlying scalp region using sponge electrodes, soaked in saline water. tDCS was done over the corresponding points of the dorsolateral prefrontal cortex (DLPFC), specifically F3/F4, and the primary motor cortex (PMC), i.e. C3/C4 of the affected hemisphere. The tDCS montage was selected based on the 10-20 EEG international classification system. Stimulation was administered at the intensity of 2 mA for 20 minutes, five sessions per week for a total of four weeks [8] (Figure 2).
Sham M-tDCS procedure
Participants in the control group were seated in a comfortable position, and all contraindications for the application of tDCS were confirmed. The placement of tDCS electrodes was done exactly as in the experimental group. The 10-20 EEG electrode placement method was followed, with electrodes positioned over (C3/C4) and (F3/F4) points corresponding to the motor cortex and dorsolateral prefrontal cortex. tDCS was initiated at an intensity of 1.2 mA, which was then reduced to zero after 30 seconds from the start of stimulation. The electrodes were kept in their respective places for 20 minutes.
Controlled intervention
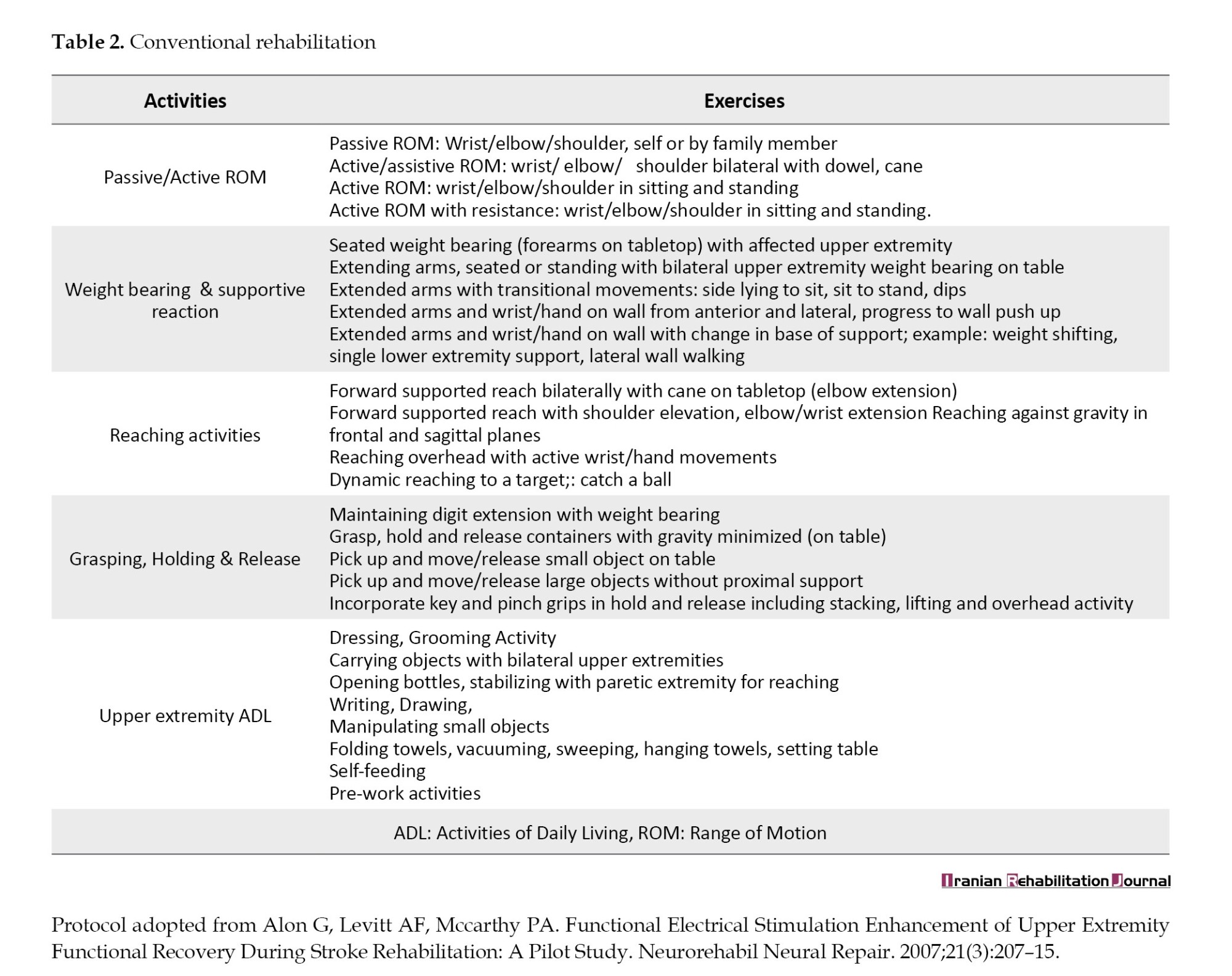
Participants of the experimental and control groups received standard physical therapy rehabilitation in addition to a) SaeboFlex training for hand and b) Bank of exercises (Table 2).
Saeboflex training
This is a movable hand and digit orthosis customized with a fabricated wrist support. It includes a forearm support fixed to a dorsal hand platform, which also features spring attachments. Each participant wore this orthosis on their paretic side and was instructed to perform various tasks involving grasping and picking up a sponge ball (7.6 cm in diameter, weighing <60 g).
The activities included were: a) Participant sat in a position and were instructed to shift the ball from their paretic side foot to the table, b) Following a diagonal pattern, participants were told to shift the ball from the unaffected side to the paretic side, c) The previous activity was repeated in a standing position, d) In the standing position, participants were instructed to shift the ball on a table from left to right, e) In the standing position, participants shifted the ball from right to left on table, f) Grasping and releasing the ball from front to back on the table, g) Grasping and releasing the ball from front to back on the table, h) Grasping and releasing the ball following a diagonal pattern on a table while standing, i) Shifting the ball between two cups placed on a table. Each activity was done for 5 to 6 minutes [9].
Primary outcome measures
The primary outcome measures of the present study were the Fugl-Meyer assessment (FMA) score and the Montreal cognitive assessment (MCA) score.
Fugl-meyer assessment (FMA)
This is an impairment index that is used for the assessment of the physical performance of stroke survivors. It has four domains, including motor domain, sensory domain, joint range of motion, and joint pain and balance. A three-point scale was used to score each domain [10].
Montreal cognitive assessment (MCA)
It was used to examine the cognitive functions of stroke survivors. It is a quick assessment tool that examines various cognitive domains, like memory (immediate memory), attention, language, visuospatial characteristics and concentration, and spatial and temporal orientation. The cumulative score ranges between 0 and 30. The maximum score indicates the good cognitive state of an individual [11].
Secondary outcomes
Nine-hole peg test (NHPT)
This test is a time-based test used to quantify the dexterity performance of individuals. The test requires a square board having nine holes and nine pegs. Participants were directed to pick pegs one by one from the container and put them in the holes as quickly as possible and then remove those pegs and put them back in the container. The time is measured in seconds [12].
Stroke-specific quality of life
This scale was used to measure the quality of life of stroke survivors. It is a standard valid scale, with a reliability coefficient of 0.92 that consists of 12 different items. Each item is grouped into subscales. There are two subscales based on the physical domain and psychological domain, with a total of 49 items. Each item was scored in 1-5 range. The score ranges from 49 to 245. High scores signify a good quality of life [13].
Grip strength measurement
JamarTM hand dynamometer was used to measure grip strength. Participants were positioned in an upright sitting position, relaxed and comfortable while holding the dynamometer in their paretic hand. Grip strength was measured in kilograms. Three measurements were taken, and the highest value among them was used for analysis [14].
Pinch strength measurement
For pinch strength measurement, the Jamar hydraulic Pinch Gauge was employed for each participant. The guidelines of the American Society of Hand Therapists (ASHT) were used to determine the participant’s position. Pinch strength was documented in kilograms for the key pinch, tip to tip pinch, pulp pinch, and three jaw chuck pinch of the affected hand. three separate trials were conducted, and the participant’s highest score was recorded [15].
Results
Statistical analysis
In the present trial, an independent researcher performed the data management and statistical analysis. The independent researcher was blind to all the group allocations. Descriptive statistics were computed for the outcome measures relevant to the study. Depending on the type of variables, differences between groups were evaluated using chi-square and student t-tests. SPSS, software, version 20 Windows 10 home edition was used to analyze the collected data in this study. An independent t-test was conducted to compare the demographic characteristics of the participants between the two groups. To minimize type 1 error, the alpha value was kept <0.05. Within the intervention group and the control group, analyses were performed using the one-way analysis of variance (ANOVA) method. The comparison between the two groups was conducted using an independent t-test [16].
Baseline characteristics
Baseline characteristics included demographic profile, and clinical characteristics of the participants. Demographic profile included Age and gender distribution of the participants and clinical characteristics included MMSE and NIHSS Scores as illustrated in the Table 3.
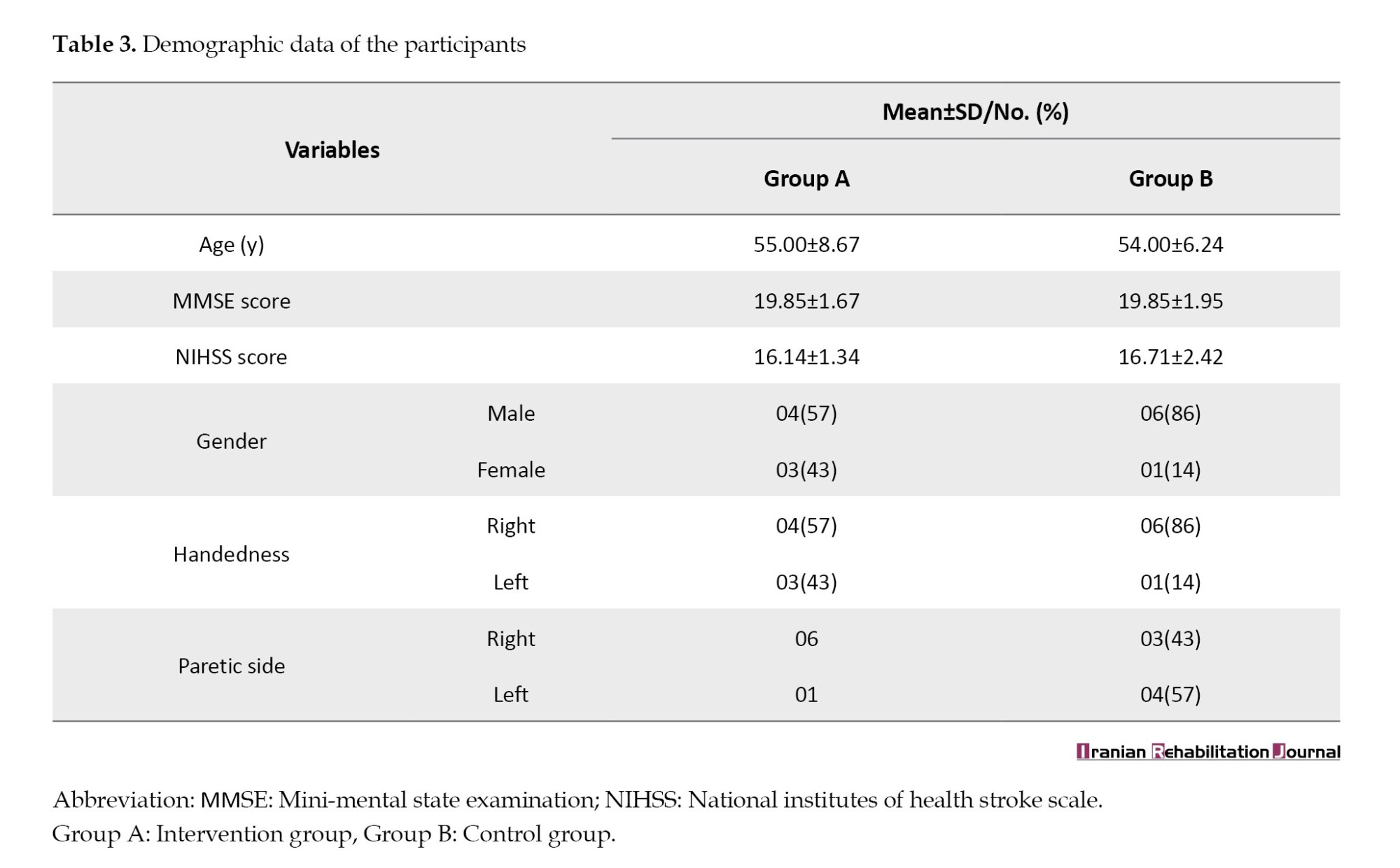
In this trial, a total of 14 participants were recruited with n=7 in each group. No statistically significant difference was found between participants’ characteristics and the flow of the trial between the groups (P>0.05) (Table 1). The pilot trial included a total of 14 stroke survivors, randomly allocated into two groups with n=7 in each group. The mean age of participants in group A was 55.00±8.67 and in group B was 54.00±6.24 years. Initial mental screening of participants in both groups was conducted using MMSE. The mean MMSE score of the experimental group was 19.85±1.67 and that of the control group participants was 19.85±1.95. The NIHSS was applied to find the stroke severity. The NIHSS score for participants in the experimental group was 16.14±1.34, and for the control group, it was 16.71±2.42. Demographic variables also included the distribution of participants based on their gender, dominance, and paretic side. Additional information about the descriptive characteristics is provided in Table 3.
Effects of the interventions
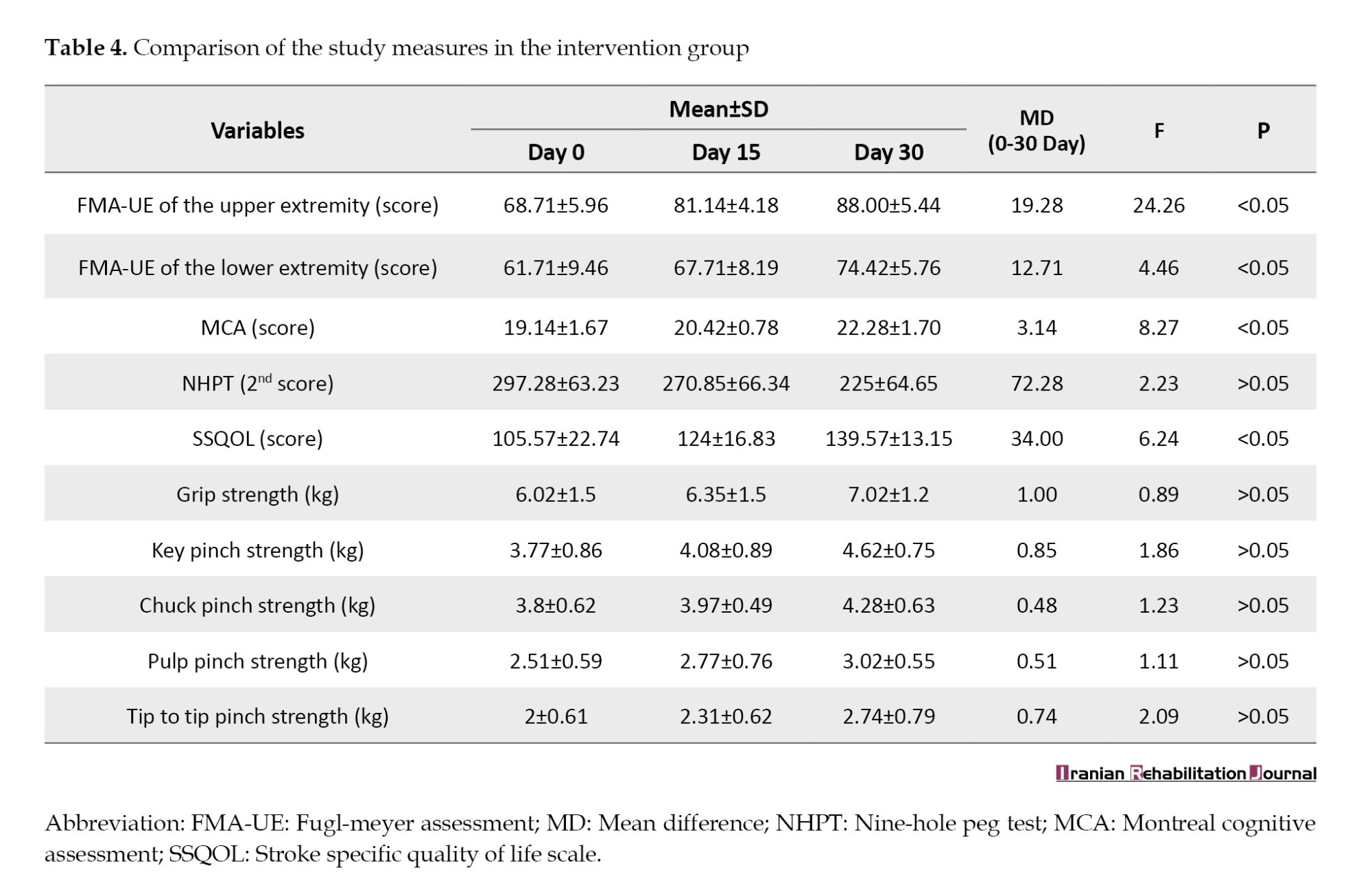
Within the intervention group, the comparison has shown significant results for Fugl-Meyer assessment of the upper extremity (FMA-UE) (F=24.26), Fugl-Meyer assessment of the lower extremity (FMA-LE) (F=4.46), MOCA (F=8.27), and SSQOL (F=6.24) at P<0.05. However, non-significant findings were observed for the outcome measures used to assess hand function, including NHPT (F=2.23), grip strength (F=0.89), chuck pinch strength (F=1.23), key pinch strength (F=1.86), Tip to Tip pinch strength (F=2.09), and pulp pinch strength (F=1.11) (P≥0.05) (Table 4).
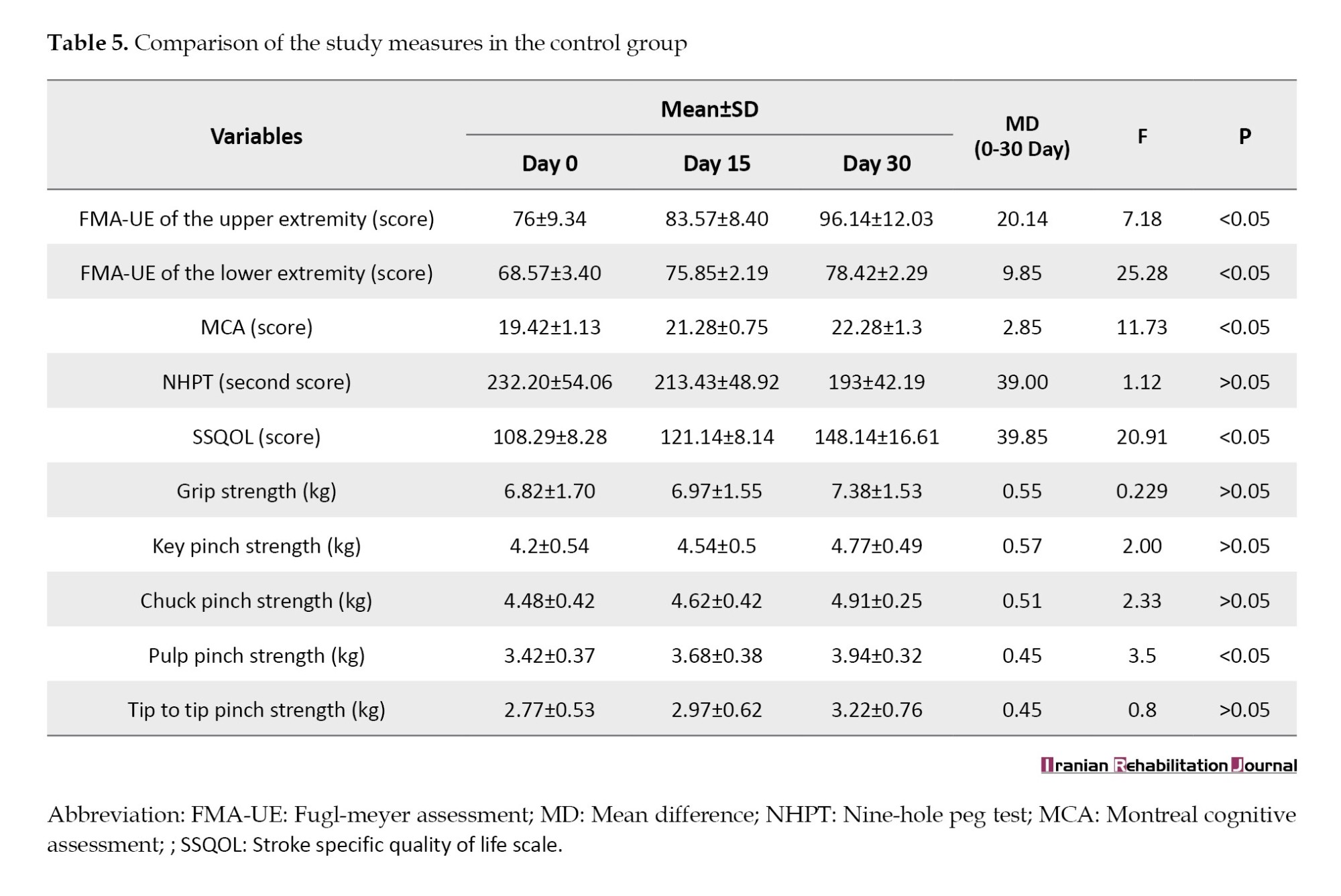
Within the control group, the comparison has shown significant results for FMA-UE (F=7.18), FMA-LE (F=25.28), MOCA (F=11.73), SSQOL (F=20.91), and pulp pinch strength (F=3.5) with P<0.05. However, non-significant results were observed for the outcome measures related to hand function assessment, including NHPT (F=1.12), grip strength (F=0.22), chuck pinch strength (F=2.33), key pinch strength (F=2.00), and tip to tip pinch strength (F=0.87) (P≥0.05) (Table 5).
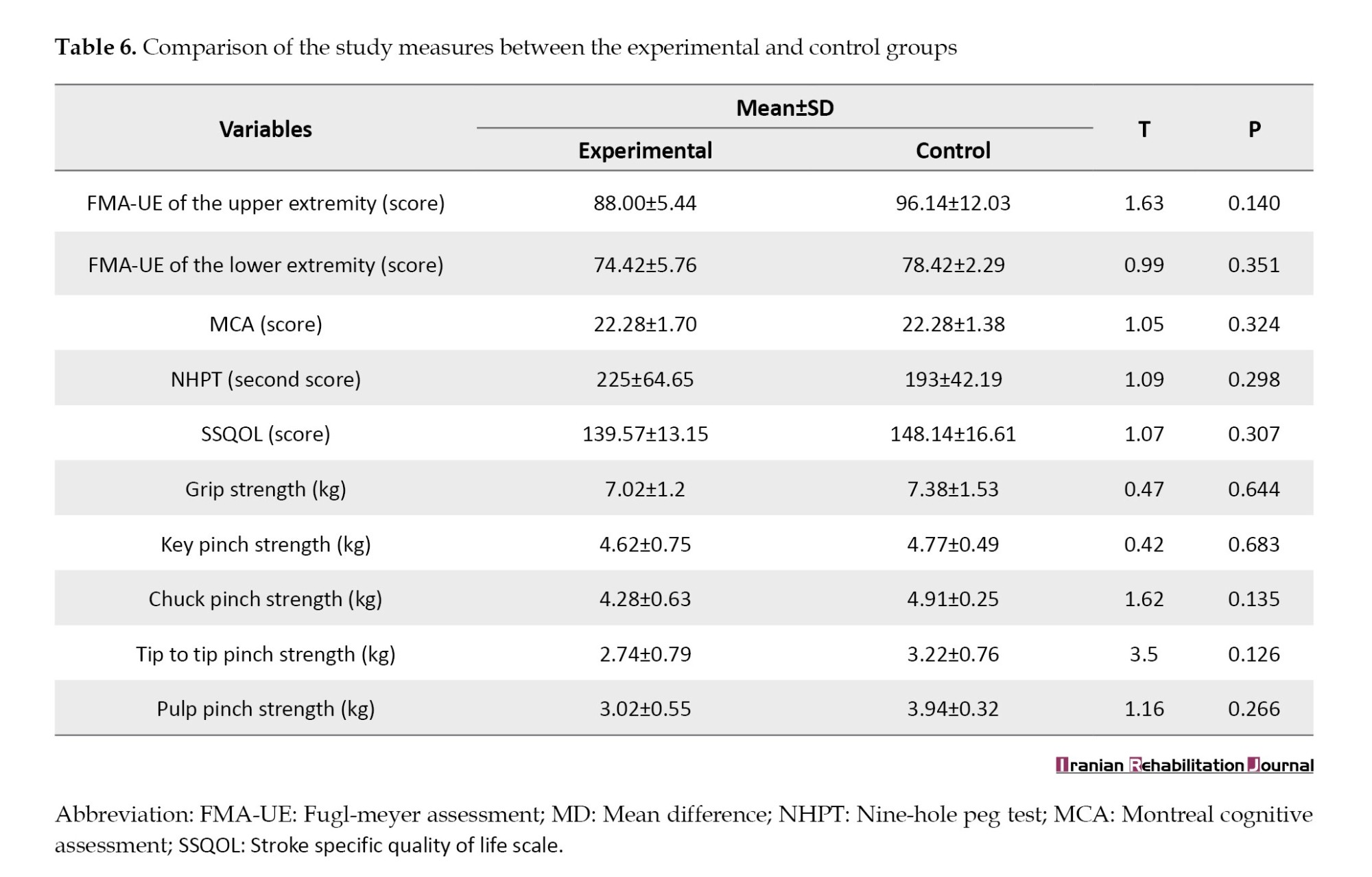
The intergroup comparison showed non- significant results for each outcome variable i.e. FMA-UE (t=1.63, P=0.140), FMA-LE (t=0.99, P=0.35), MOCA(t=1.05, P=0.32), SSQOL (t=1.07, P=0.30), NHPT (t=1.09, P=0.29), grip strength (t=0.47, P=0.64), chuck pinch strength (t=1.62, P=0.13), key pinch strength (t=0.42, P=0.68), pulp pinch strength (t=1.16, P=0.26), and tip to tip pinch strength (t=3.5, P=0.12) (Table 6).
Discussion
A total of 14 participants were recruited in the present trial. Participants were randomly allocated into two groups, with n=07 in each group. In the experimental group, participants received intervention with multichannel tDCS, FES application on the affected lower limb, and exercises. They also wore the Saebo Flex on the affected hand during exercises and underwent standard physical therapy rehabilitation. In the control group, participants received sham M-tDCS and the rest of the intervention was the same as that given to the experimental group.
The motor cortex controls the movements of individual digits and also coordinates the synergy of multiple digits. The cortical organization does not have separate areas for the representation of each digit; hence, it is difficult to achieve individual movements of single digits and much easier to move multiple digits together [17, 18]. Following a stroke, even when sufficient strength recovers in the upper extremity, the relative lack of individual digit movement makes fine manipulation difficult for the survivors, and this deficit persists forever [19].
The recovery following a stroke is a plasticity-dependent process. Non-invasive brain stimulation intervention, i.e. tDCS is one of the effective therapies used as an adjunct to stroke rehabilitation. It is a neuromodulation method to modulate the brain plasticity. What sets the present trial apart is the provision of M-tDCS in addition to physiotherapy interventions using SaeboFlex and standard physical therapy rehabilitation for subacute stroke survivors.
The concurrent presence of motor and cognitive deficits among stroke survivors has a negative effect on their daily routines. To the best of the researcher’s knowledge, most clinical trials have focused on either motor deficits or cognitive deficits separately [20]. Therefore, the authors of this trial made an attempt to establish the efficacy of M-tDCS while targeting both motor and cognitive cortices simultaneously, alongside physiotherapy interventions using SaeboFlex and conventional care. Peripheral stimulation was also administered to enhance the positive impact created by the M-tDCS application.
The comparative analysis of the change scores between the experimental and control groups revealed significant findings for NHPT and grip strength, whereas non-significant findings were observed for the rest of the outcome measures. The non-significant findings might be attributed to certain factors that hinder the full integration of M-tDCS stroke rehabilitation. The first factor is the small sample size in the present study, where 14 participants were selected with n=7 in each group. The second factor could be the heterogeneity of participants, like the type of lesion, post-stroke severity, and anatomical factors, like the head size of the participants, which could have limited the efficacy of the intervention.
The results of this trial were similar to those of Heller et al. In their study, significant changes were seen in the grip strength and ability to manipulate objects [18]. Similarly, in another study, grip strength improved significantly compared to the dexterity function of the hand. They concluded that it is a very common finding to see significant improvement in grip strength, which helps stroke survivors complete power grip tasks, even though they may still struggle to move individual digits [21].
Experimental group intervention
The within-group analysis of the experimental group revealed significant findings for all the outcome measures of the trial. To the best of the investigator’s knowledge, there is no study available, in which the effect of M-tDCS has been investigated on motor recovery in stroke. Most of the existing literature focuses on conditions such as disorders of consciousness and Parkinson’s disease [5]. The findings of the present study are similar to the study done on Parkinson’s patients, where improvement was observed in the motor function of the lower extremities [5]. However, there is a lack of literature on the application of M-tDCS in the stroke population.
Control group intervention
The participants in the control group received sham M-tDCS, in which the stimulation intensity was dropped to zero 30 seconds after it started, along with peripheral stimulation using Saeboflex training and standard physiotherapy care. The within-group analysis of the experimental group revealed significant findings for all the outcome measures of the trial. The findings of this trial were similar to a trial done by Franck et al. who stated that individuals with subacute stroke show great improvement in their functional activities and activities of daily living with appropriate physiotherapy care and use of arm orthosis [21]. The Saeboflex orthosis facilitates quick training of grasp and release activities, which improves the motor function of the upper limb. It consists of a wrist orthosis that supports the paretic hand, with a spiral forearm support attached to the dorsum of the affected hand, along with two spring attachments. Individual finger sleeves are connected to the springs to provide support for finger extension [22].
Safety and adverse effects following M-tDCS
During the course of the study, no potential adverse effects were reported by the participants during and immediately after the application of M-tDCS. However, transient side effects in the form of mild itching, lack of sleep, and mild redness were observed in some participants. These side effects did not pose any medical emergency and indicate the safe applicability and tolerability of M-tDCS at an intensity of 1.2 mA when NIBS guidelines are appropriately followed [23].
Future scope
Large-scale trials are needed with participants who have UCP to establish the effects of M-tDCS on motor recovery in the upper extremities. Different trials could also be conducted by combining multiple sessions of anodal tDCS with motor training. There is significant scope for trials aimed at establishing various parameters, like tDCS dosage, stimulation timing, and montage in different age groups.
Conclusion
The current study concludes that there was a significant improvement in grip strength between the two groups, but no improvement was observed in the individuation of digits in either group. The mean scores of all the outcome measures changed, indicating clinical improvement in the proximal joints.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by Research Ethics Committee of Punjabi University (No.: 152/IEC-2019). Initial screening of all the participants was done with a population screening form, and the assessment was conducted using the “performance of comprehensive neurological assessment” by the Copyright Office of the Government of India (No.: L-80961/2019). The trial was registered at the clinical trial registry of India (CTRI) (Code: CTRI/2020/01/022998, dated 24/01/2020).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the Physiotherapy Department for providing resources for the trial and Medicaid India for giving equipment support.
References
Stroke has emerged as a major public health concern in the past decade, and its prevalence may continue to rise, especially in developing countries due to shifts in demographic profiles. Hence, stroke occupies a prominent position on the agenda of various health-related issues facing the general public in the 21st century. It represents an important area for general health research, necessitating robust interventions aimed at achieving high survival rates and enhancing the quality of life for stroke survivors [1]. Among the varied symptoms, motor impairment ranks as one of the most prevalent deficits in stroke survivors (PABLO) [2], making it a major contributor to physical and mental disability among them. Motor recovery following stroke is one of the key concerns of rehabilitation professionals.
To achieve optimal recovery and develop effective intervention strategies for compensating for brain injury, it is essential to harness the intrinsic capacity of viable cortical networks. However, despite the implementation of novel therapeutic approaches, the effect size of individual interventions may sometimes be insufficient to induce complete recovery [3].
The human brain is a complex network of multiple cortical networks. Brain stimulation modulates network activity. However, targeting a single area of the brain may not yield satisfactory results [4]. The restoration of motor functions relies on the viability of various circuits connecting the hemispheres, with motor networks closely interacting with nearby cognitive networks. Hence, the influence of cognitive networks on motor recovery is significant and cannot be overlooked. Motor learning exhibits a strong association with various cognitive domains. The same principle can be applied by targeting more than one corresponding neuronal circuit in the cerebral cortex via neuro-modulation [5].
Neuro-modulation is achieved by applying a weak electric current, which causes the activation or deactivation of excitable tissue, leading to improved patient outcomes. Transcranial direct current stimulation (tDCS) is one of the key neuromodulation tools with therapeutic benefits in post-stroke rehabilitation. Many authors have successfully used tDCS in their studies. However, in most of the trials, the authors provided tDCS over a single cortical region at different time periods to get the desired outcome [6]. Some authors have also stated that tDCS may have better results with a better understanding of neural networks and their connections with adjacent regions along with conventional rehabilitation. Hence, the aim of the current trial was to investigate the effect of multichannel transcranial direct current stimulation (M-tDCS) on global recovery following stroke.
Materials and Methods
Participant screening and recruitment
The present study was a prospective, parallel-group, double-blinded randomized controlled trial. Stroke survivors in the age range of 40-75 years were selected from neuro-physiotherapy rehabilitation units/centers in Patiala City of Punjab, India from January 2020 to December 2021. Thirty-five individuals were screened, of whom 14 individuals fulfilled the eligibility criteria of the study. The participation schedule was prepared according to the guidelines of intervention trials (Table 1) [7].

Eligibility Criteria
Inclusion criteria
Participants were selected as per the following criteria: Both male and female individuals, medically diagnosed with cortical stroke (ischemic), age between 40 and 75 years, preserved range of motion of the wrist (approximately 10 degrees), modified Ashworth scale grade <2 (in major muscle groups in the upper extremity and lower extremity), mini-mental state examination (MMSE) score between 18-23, and those with ambulatory care.
Exclusion criteria
Individuals who did not meet the inclusion criteria were excluded from the study. Exclusion criteria included:
Medical diagnosis of hemorrhagic stroke; history of neurological disorders other than stroke; musculoskeletal disorders affecting upper or lower extremity motor function; visual analog scale score exceeding 4 in the upper or lower extremity; individuals with psychosomatic illnesses; medically unstable individuals with a history of cardiovascular or respiratory illnesses; systemic illnesses; presence of metallic implants; pregnancy; uncontrolled hypertension; previous participation in any other pharmacological or rehabilitation study; sensory disorders; lack of interest in participating in the study.
Participant allocation
All subjects received participant information sheets in their local language and written consent was taken from them before the study. Complete information was given to participants about the objectives of the study, study procedure, and major/minor risks and/or benefits. Participants were also told that their enrolment was entirely voluntary and that they had the full right to withdraw from the study at any stage.
Randomization, allocation, and blinding
Out of 35 individuals, 14 participants were recruited for the trial. The investigator prepared a randomization schedule before the start of the study using a computer-generated random list of participants. Random allocation of participants was done, with 7 individuals in each group. The computer-generated list was sealed in an opaque envelope and given to a third person who had no direct or indirect involvement in the study. The participants and assessors were blinded to the intervention and allocation throughout the entire study process. The schematic consolidated standard of reporting trials flow chart for the study protocol is shown in Figure 1.

Enrolment and baseline measurements and clinical trial registration
The demographic profile of the participants included their names, age, and gender. Baseline data of all the participants were documented, which included their mechanism of stroke, stroke duration, hemiparetic side, participant handedness, MMSE score, and the National Institutes of Health Stroke Scale (NIHSS) score. The Standard protocol items: Recommendations for Interventional Trials (SPIRIT) statement was used for documenting the schedule of study participants. The enrolment process was initiated from January 2020 onwards.
Interventions
M-tDCS procedure
Participants in the experimental group were given M-tDCS on the underlying scalp region using sponge electrodes, soaked in saline water. tDCS was done over the corresponding points of the dorsolateral prefrontal cortex (DLPFC), specifically F3/F4, and the primary motor cortex (PMC), i.e. C3/C4 of the affected hemisphere. The tDCS montage was selected based on the 10-20 EEG international classification system. Stimulation was administered at the intensity of 2 mA for 20 minutes, five sessions per week for a total of four weeks [8] (Figure 2).

Sham M-tDCS procedure
Participants in the control group were seated in a comfortable position, and all contraindications for the application of tDCS were confirmed. The placement of tDCS electrodes was done exactly as in the experimental group. The 10-20 EEG electrode placement method was followed, with electrodes positioned over (C3/C4) and (F3/F4) points corresponding to the motor cortex and dorsolateral prefrontal cortex. tDCS was initiated at an intensity of 1.2 mA, which was then reduced to zero after 30 seconds from the start of stimulation. The electrodes were kept in their respective places for 20 minutes.
Controlled intervention
Participants of the experimental and control groups received standard physical therapy rehabilitation in addition to a) SaeboFlex training for hand and b) Bank of exercises (Table 2).

Saeboflex training
This is a movable hand and digit orthosis customized with a fabricated wrist support. It includes a forearm support fixed to a dorsal hand platform, which also features spring attachments. Each participant wore this orthosis on their paretic side and was instructed to perform various tasks involving grasping and picking up a sponge ball (7.6 cm in diameter, weighing <60 g).
The activities included were: a) Participant sat in a position and were instructed to shift the ball from their paretic side foot to the table, b) Following a diagonal pattern, participants were told to shift the ball from the unaffected side to the paretic side, c) The previous activity was repeated in a standing position, d) In the standing position, participants were instructed to shift the ball on a table from left to right, e) In the standing position, participants shifted the ball from right to left on table, f) Grasping and releasing the ball from front to back on the table, g) Grasping and releasing the ball from front to back on the table, h) Grasping and releasing the ball following a diagonal pattern on a table while standing, i) Shifting the ball between two cups placed on a table. Each activity was done for 5 to 6 minutes [9].
Primary outcome measures
The primary outcome measures of the present study were the Fugl-Meyer assessment (FMA) score and the Montreal cognitive assessment (MCA) score.
Fugl-meyer assessment (FMA)
This is an impairment index that is used for the assessment of the physical performance of stroke survivors. It has four domains, including motor domain, sensory domain, joint range of motion, and joint pain and balance. A three-point scale was used to score each domain [10].
Montreal cognitive assessment (MCA)
It was used to examine the cognitive functions of stroke survivors. It is a quick assessment tool that examines various cognitive domains, like memory (immediate memory), attention, language, visuospatial characteristics and concentration, and spatial and temporal orientation. The cumulative score ranges between 0 and 30. The maximum score indicates the good cognitive state of an individual [11].
Secondary outcomes
Nine-hole peg test (NHPT)
This test is a time-based test used to quantify the dexterity performance of individuals. The test requires a square board having nine holes and nine pegs. Participants were directed to pick pegs one by one from the container and put them in the holes as quickly as possible and then remove those pegs and put them back in the container. The time is measured in seconds [12].
Stroke-specific quality of life
This scale was used to measure the quality of life of stroke survivors. It is a standard valid scale, with a reliability coefficient of 0.92 that consists of 12 different items. Each item is grouped into subscales. There are two subscales based on the physical domain and psychological domain, with a total of 49 items. Each item was scored in 1-5 range. The score ranges from 49 to 245. High scores signify a good quality of life [13].
Grip strength measurement
JamarTM hand dynamometer was used to measure grip strength. Participants were positioned in an upright sitting position, relaxed and comfortable while holding the dynamometer in their paretic hand. Grip strength was measured in kilograms. Three measurements were taken, and the highest value among them was used for analysis [14].
Pinch strength measurement
For pinch strength measurement, the Jamar hydraulic Pinch Gauge was employed for each participant. The guidelines of the American Society of Hand Therapists (ASHT) were used to determine the participant’s position. Pinch strength was documented in kilograms for the key pinch, tip to tip pinch, pulp pinch, and three jaw chuck pinch of the affected hand. three separate trials were conducted, and the participant’s highest score was recorded [15].
Results
Statistical analysis
In the present trial, an independent researcher performed the data management and statistical analysis. The independent researcher was blind to all the group allocations. Descriptive statistics were computed for the outcome measures relevant to the study. Depending on the type of variables, differences between groups were evaluated using chi-square and student t-tests. SPSS, software, version 20 Windows 10 home edition was used to analyze the collected data in this study. An independent t-test was conducted to compare the demographic characteristics of the participants between the two groups. To minimize type 1 error, the alpha value was kept <0.05. Within the intervention group and the control group, analyses were performed using the one-way analysis of variance (ANOVA) method. The comparison between the two groups was conducted using an independent t-test [16].
Baseline characteristics
Baseline characteristics included demographic profile, and clinical characteristics of the participants. Demographic profile included Age and gender distribution of the participants and clinical characteristics included MMSE and NIHSS Scores as illustrated in the Table 3.

In this trial, a total of 14 participants were recruited with n=7 in each group. No statistically significant difference was found between participants’ characteristics and the flow of the trial between the groups (P>0.05) (Table 1). The pilot trial included a total of 14 stroke survivors, randomly allocated into two groups with n=7 in each group. The mean age of participants in group A was 55.00±8.67 and in group B was 54.00±6.24 years. Initial mental screening of participants in both groups was conducted using MMSE. The mean MMSE score of the experimental group was 19.85±1.67 and that of the control group participants was 19.85±1.95. The NIHSS was applied to find the stroke severity. The NIHSS score for participants in the experimental group was 16.14±1.34, and for the control group, it was 16.71±2.42. Demographic variables also included the distribution of participants based on their gender, dominance, and paretic side. Additional information about the descriptive characteristics is provided in Table 3.
Effects of the interventions
Within the intervention group, the comparison has shown significant results for Fugl-Meyer assessment of the upper extremity (FMA-UE) (F=24.26), Fugl-Meyer assessment of the lower extremity (FMA-LE) (F=4.46), MOCA (F=8.27), and SSQOL (F=6.24) at P<0.05. However, non-significant findings were observed for the outcome measures used to assess hand function, including NHPT (F=2.23), grip strength (F=0.89), chuck pinch strength (F=1.23), key pinch strength (F=1.86), Tip to Tip pinch strength (F=2.09), and pulp pinch strength (F=1.11) (P≥0.05) (Table 4).

Within the control group, the comparison has shown significant results for FMA-UE (F=7.18), FMA-LE (F=25.28), MOCA (F=11.73), SSQOL (F=20.91), and pulp pinch strength (F=3.5) with P<0.05. However, non-significant results were observed for the outcome measures related to hand function assessment, including NHPT (F=1.12), grip strength (F=0.22), chuck pinch strength (F=2.33), key pinch strength (F=2.00), and tip to tip pinch strength (F=0.87) (P≥0.05) (Table 5).

The intergroup comparison showed non- significant results for each outcome variable i.e. FMA-UE (t=1.63, P=0.140), FMA-LE (t=0.99, P=0.35), MOCA(t=1.05, P=0.32), SSQOL (t=1.07, P=0.30), NHPT (t=1.09, P=0.29), grip strength (t=0.47, P=0.64), chuck pinch strength (t=1.62, P=0.13), key pinch strength (t=0.42, P=0.68), pulp pinch strength (t=1.16, P=0.26), and tip to tip pinch strength (t=3.5, P=0.12) (Table 6).

Discussion
A total of 14 participants were recruited in the present trial. Participants were randomly allocated into two groups, with n=07 in each group. In the experimental group, participants received intervention with multichannel tDCS, FES application on the affected lower limb, and exercises. They also wore the Saebo Flex on the affected hand during exercises and underwent standard physical therapy rehabilitation. In the control group, participants received sham M-tDCS and the rest of the intervention was the same as that given to the experimental group.
The motor cortex controls the movements of individual digits and also coordinates the synergy of multiple digits. The cortical organization does not have separate areas for the representation of each digit; hence, it is difficult to achieve individual movements of single digits and much easier to move multiple digits together [17, 18]. Following a stroke, even when sufficient strength recovers in the upper extremity, the relative lack of individual digit movement makes fine manipulation difficult for the survivors, and this deficit persists forever [19].
The recovery following a stroke is a plasticity-dependent process. Non-invasive brain stimulation intervention, i.e. tDCS is one of the effective therapies used as an adjunct to stroke rehabilitation. It is a neuromodulation method to modulate the brain plasticity. What sets the present trial apart is the provision of M-tDCS in addition to physiotherapy interventions using SaeboFlex and standard physical therapy rehabilitation for subacute stroke survivors.
The concurrent presence of motor and cognitive deficits among stroke survivors has a negative effect on their daily routines. To the best of the researcher’s knowledge, most clinical trials have focused on either motor deficits or cognitive deficits separately [20]. Therefore, the authors of this trial made an attempt to establish the efficacy of M-tDCS while targeting both motor and cognitive cortices simultaneously, alongside physiotherapy interventions using SaeboFlex and conventional care. Peripheral stimulation was also administered to enhance the positive impact created by the M-tDCS application.
The comparative analysis of the change scores between the experimental and control groups revealed significant findings for NHPT and grip strength, whereas non-significant findings were observed for the rest of the outcome measures. The non-significant findings might be attributed to certain factors that hinder the full integration of M-tDCS stroke rehabilitation. The first factor is the small sample size in the present study, where 14 participants were selected with n=7 in each group. The second factor could be the heterogeneity of participants, like the type of lesion, post-stroke severity, and anatomical factors, like the head size of the participants, which could have limited the efficacy of the intervention.
The results of this trial were similar to those of Heller et al. In their study, significant changes were seen in the grip strength and ability to manipulate objects [18]. Similarly, in another study, grip strength improved significantly compared to the dexterity function of the hand. They concluded that it is a very common finding to see significant improvement in grip strength, which helps stroke survivors complete power grip tasks, even though they may still struggle to move individual digits [21].
Experimental group intervention
The within-group analysis of the experimental group revealed significant findings for all the outcome measures of the trial. To the best of the investigator’s knowledge, there is no study available, in which the effect of M-tDCS has been investigated on motor recovery in stroke. Most of the existing literature focuses on conditions such as disorders of consciousness and Parkinson’s disease [5]. The findings of the present study are similar to the study done on Parkinson’s patients, where improvement was observed in the motor function of the lower extremities [5]. However, there is a lack of literature on the application of M-tDCS in the stroke population.
Control group intervention
The participants in the control group received sham M-tDCS, in which the stimulation intensity was dropped to zero 30 seconds after it started, along with peripheral stimulation using Saeboflex training and standard physiotherapy care. The within-group analysis of the experimental group revealed significant findings for all the outcome measures of the trial. The findings of this trial were similar to a trial done by Franck et al. who stated that individuals with subacute stroke show great improvement in their functional activities and activities of daily living with appropriate physiotherapy care and use of arm orthosis [21]. The Saeboflex orthosis facilitates quick training of grasp and release activities, which improves the motor function of the upper limb. It consists of a wrist orthosis that supports the paretic hand, with a spiral forearm support attached to the dorsum of the affected hand, along with two spring attachments. Individual finger sleeves are connected to the springs to provide support for finger extension [22].
Safety and adverse effects following M-tDCS
During the course of the study, no potential adverse effects were reported by the participants during and immediately after the application of M-tDCS. However, transient side effects in the form of mild itching, lack of sleep, and mild redness were observed in some participants. These side effects did not pose any medical emergency and indicate the safe applicability and tolerability of M-tDCS at an intensity of 1.2 mA when NIBS guidelines are appropriately followed [23].
Future scope
Large-scale trials are needed with participants who have UCP to establish the effects of M-tDCS on motor recovery in the upper extremities. Different trials could also be conducted by combining multiple sessions of anodal tDCS with motor training. There is significant scope for trials aimed at establishing various parameters, like tDCS dosage, stimulation timing, and montage in different age groups.
Conclusion
The current study concludes that there was a significant improvement in grip strength between the two groups, but no improvement was observed in the individuation of digits in either group. The mean scores of all the outcome measures changed, indicating clinical improvement in the proximal joints.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by Research Ethics Committee of Punjabi University (No.: 152/IEC-2019). Initial screening of all the participants was done with a population screening form, and the assessment was conducted using the “performance of comprehensive neurological assessment” by the Copyright Office of the Government of India (No.: L-80961/2019). The trial was registered at the clinical trial registry of India (CTRI) (Code: CTRI/2020/01/022998, dated 24/01/2020).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the Physiotherapy Department for providing resources for the trial and Medicaid India for giving equipment support.
References
- Donkor ES. Stroke in the 21st century: A Snapshot of the burden, epidemiology, and quality of life. Stroke Research and Treatment. 2018; 2018:3238165. [DOI:10.1155/2018/3238165] [PMID] [PMCID]
- Aqueveque P, Ortega P, Pino E, Saavedra F, Germany E, Gómez B. After stroke movement impairments: A review of current technologies for rehabilitation. Physical Disabilities-Therapeutic Implications. 2017; 10:95-116. [DOI:10.5772/67577]
- Goodwill AM, Teo WP, Morgan P, Daly RM, Kidgell DJ. Bihemispheric-tDCS and upper limb rehabilitation improves retention of motor function in chronic stroke: A pilot study. Frontiers in Human Neuroscience. 2016; 10:258. [DOI:10.3389/fnhum.2016.00258] [PMID] [PMCID]
- Bao SC, Khan A, Song R, Kai-Yu Tong R. Rewiring the lesioned brain: Electrical stimulation for post-stroke motor restoration. Journal of Stroke. 2020; 22(1):47-63. [DOI:10.5853/jos.2019.03027] [PMID] [PMCID]
- Dagan M, Herman T, Harrison R, Zhou J, Giladi N, Ruffini G, et al. Multitarget transcranial direct current stimulation for freezing of gait in Parkinson's disease. Movement Disorders. 2018; 33(4):642-6. [DOI:10.1002/mds.27300] [PMID] [PMCID]
- Bolognini N, Pascual-Leone A, Fregni F. Using non-invasive brain stimulation to augment motor training-induced plasticity. Journal of Neuroengineering and Rehabilitation. 2009; 6:8. [DOI:10.1186/1743-0003-6-8] [PMID] [PMCID]
- SPIRIT. SPIRIT (standard protocol items: Recommendations for interventional trials) [Internet]. 2019 [Updated 2020 February 2]. Available from: [Link]
- Otal B, Dutta A, Foerster Á, Ripolles O, Kuceyeski A, Miranda PC, et al. Opportunities for guided multichannel non-invasive transcranial current stimulation in poststroke rehabilitation. Frontiers in Neurology. 2016;7:21. [DOI: 10.3389/fneur.2016.00021] [PMID]
- Woo Y, Jeon H, Hwang S, Choi B, Lee J. Kinematics variations after spring-assisted orthosis training in persons with stroke. Prosthetics and Orthotics International. 2013; 37(4):311-6. [DOI:10.1177/0309364612461050] [PMID]
- Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabilitation and Neural Repair. 2002; 16(3):232-40. [DOI:10.1177/154596802401105171] [PMID]
- Hobson J. The montreal cognitive assessment (MoCA). Occupational Medicine. 2015; 65(9):764-5. [DOI:10.1093/occmed/kqv078] [PMID]
- Sarikaya PM, Incel NA, Yilmaz A, Cimen OB, Sahin G. Effect of hand dominance on functional status and recovery of hand in stroke patients. Science. 2017; 6(3):39-45. [DOI:10.11648/j.sjcm.20170603.12]
- de Souza JA, Corrêa JCF, Agnol LD, Dos Santos FR, Gomes MRP, Corrêa FI. Effects of transcranial direct current stimulation on the rehabilitation of painful shoulder following a stroke: protocol for a randomized, controlled, double-blind, clinical trial. Trials. 2019; 20(1):165. [DOI:10.1186/s13063-019-3266-y] [PMID] [PMCID]
- Sheorajpanday RV, Nagels G, Weeren AJ, van Putten MJ, De Deyn PP. Quantitative EEG in ischemic stroke: Correlation with functional status after 6 months. Clinical Neurophysiology. 2011; 122(5):874-83. [DOI:10.1016/j.clinph.2010.07.028] [PMID]
- Walukonis K, Beasley J, Boerema R, Powers J, Anderson K. The impact of finger position on pinch strength. Hand Therapy. 2018; 23(2):70-6. [DOI:10.1177/1758998317752966]
- Vickers NJ. Animal communication: When i'm calling you, will you answer too? Current Biology. 2017; 27(14):R713-5. [DOI:10.1016/j.cub.2017.05.064] [PMID]
- Feng W, Kautz SA, Schlaug G, Meinzer C, George MS, Chhatbar PY. Transcranial direct current stimulation for poststroke motor recovery: Challenges and opportunities. PM & R. 2018; 10(9 Suppl 2):S157-64. [DOI:10.1016/j.pmrj.2018.04.012] [PMID] [PMCID]
- Heller A, Wade DT, Wood VA, Sunderland A, Hewer RL, Ward E. Arm function after stroke: Measurement and recovery over the first three months. Journal of Neurology, Neurosurgery, and Psychiatry. 1987; 50(6):714-9. [DOI:10.1136/jnnp.50.6.714] [PMID] [PMCID]
- Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. Journal of Neurology, Neurosurgery, and Psychiatry. 1989; 52(11):1267-72. [DOI:10.1136/jnnp.52.11.1267] [PMID] [PMCID]
- Huang Y, Thomas C, Datta A, Parra LC. Optimized tDCS for targeting multiple brain regions: An integrated implementation. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. 2018; 2018:3545-8. [DOI:10.1109/EMBC.2018.8513034] [PMID]
- Franck JA, Timmermans AA, Seelen HAM. Effects of a dynamic hand orthosis for functional use of the impaired upper limb in sub-acute stroke patients: A multiple single case experimental design study. Technology and Disability. 2013; 25(3):177-87. [DOI:10.3233/TAD-130374]
- McCombe Waller S, Whitall J, Jenkins T, Magder LS, Hanley DF, et al. Sequencing bilateral and unilateral task-oriented training versus task oriented training alone to improve arm function in individuals with chronic stroke. BMC Neurology. 2014; 14:236. [DOI:10.1186/s12883-014-0236-6] [PMID] [PMCID]
- Russo C, Souza Carneiro MI, Bolognini N, Fregni F. Safety review of transcranial direct current stimulation in stroke. Neuromodulation. 2017; 20(3):215-22. [DOI:10.1111/ner.12574] [PMID] [PMCID]
Article type: Original Research Articles |
Subject:
Neurorehabilitation
Received: 2022/12/13 | Accepted: 2023/10/11 | Published: 2024/03/1
Received: 2022/12/13 | Accepted: 2023/10/11 | Published: 2024/03/1
Send email to the article author







