Volume 22, Issue 2 (June 2024)
Iranian Rehabilitation Journal 2024, 22(2): 217-226 |
Back to browse issues page
Ethics code: IR.USWR.REC.1401.066
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Banari A, Sadeghi Z, Darouie A, Masoudian Hosseinabad N, Noroozi M. Incidence and Related Factors to Developing Dysphagia in Hospitalized COVID-19 Patients. Iranian Rehabilitation Journal 2024; 22 (2) :217-226
URL: http://irj.uswr.ac.ir/article-1-1870-en.html
URL: http://irj.uswr.ac.ir/article-1-1870-en.html
Akbar Banari1 

 , Zahra Sadeghi *1
, Zahra Sadeghi *1 

 , Akbar Darouie1
, Akbar Darouie1 

 , Niloofar Masoudian Hosseinabad1
, Niloofar Masoudian Hosseinabad1 

 , Mehdi Noroozi2
, Mehdi Noroozi2 




 , Zahra Sadeghi *1
, Zahra Sadeghi *1 

 , Akbar Darouie1
, Akbar Darouie1 

 , Niloofar Masoudian Hosseinabad1
, Niloofar Masoudian Hosseinabad1 

 , Mehdi Noroozi2
, Mehdi Noroozi2 


1- Department of Speech Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Substance Abuse and Dependence Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Substance Abuse and Dependence Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
Full-Text [PDF 787 kb]
(780 Downloads)
| Abstract (HTML) (2168 Views)
Full-Text: (603 Views)
Introduction
Coronavirus disease 2019 (COVID-19) which is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) first appeared in December 2019 in China. As the disease was highly contagious, it affected the world and started a pandemic [1]. The symptoms of this respiratory disease are diverse. Sometimes the disease can be latent without symptoms and sometimes it can threaten a person’s life, resulting in hospitalization and admission to intensive care units (ICUs) [2-4].
Common complications of hospitalized COVID-19 patients include sepsis, shock, acute respiratory distress syndrome, acute kidney failure, neurologic conditions, acute respiratory failure, acute ischemic heart disease, and venous thromboembolism [5]. COVID-19 mostly disturbs the respiratory system and can lead to acute respiratory distress syndrome [6]. These patients may require invasive respiratory support devices, such as nasogastric tube or mechanical ventilation (MV) [7]. Administering respiratory support devices may increase the risk of dysphagia in these patients [8]. Furthermore, dysphagia in COVID-19 patients results from incoordination between swallowing and breathing [9]. Medications prescribed for COVID-19, including corticosteroids which develop myopathy as a side effect, hydroxychloroquine which raises corticosteroid myopathy, and sedation and relaxation for a long period can cause swallowing impairment [10]. Dysphagia is associated with severe and multifaceted poor outcomes, such as malnutrition, aspiration pneumonia, dehydration, extended hospitalization, and raised mortality rates [9, 11]. Consequently, continued monitoring of patients for risk of dysphagia is warranted. Establishing the predictor factors of developing dysphagia will alert medical staff for careful monitoring and can contribute to the evolvement of management protocols for efficient and well-timed care that prevents its severe outcomes [8].
Swallowing is a complicated cognitive-sensorimotor process regulated by several brain areas spreading from higher cortical regions to the central pattern generator in the lateral medulla due to closely coordinated and sequential voluntary and involuntary behaviors for the anticipatory, oral preparatory, oral transit, and pharyngeal phases of the swallow [12]. Magnetoencephalography studies demonstrating the temporal features of cortical activation during intentional swallowing display the activity of the left primary sensorimotor cerebral cortex during voluntary swallowing. Further, other studies have specified that the cingulate cortex, the insula, and the inferior frontal gyrus are activated by each swallow [13]. Swallowing is a reflexive reaction and a stream of complex cognitive processes involving timely initiation of the swallowing reflex and maintaining safe swallowing [14].
The prevalence of swallowing issues in this group of patients has shown different figures, from 7% to 55% [15, 16]. Bordejé Laguna et al. conducted a study investigating the dysphagia incidence in 93 patients with severe pneumonia due to SARS-CoV-2, who required MV. The authors used the modified viscosity volume swallowing test to evaluate the swallowing status. The findings of this study showed that the incidence of dysphagia in these patients is 26.9% [10]. In a study by Rouhani et al. an eating assessment tool questionnaire was applied to a group of 41 patients who ought to have tracheostomy to treat respiratory problems caused by COVID-19. The study demonstrated that 30% of the patients had abnormal swallowing [17].
Different researchers have tried to understand the relationship between swallowing disorders and factors, for instance, demographic variables, including gender, age, comorbidities, and clinical factors, including MV variables, prone positioning, sedative agents, ICU, and hospital long of stay (LOS) [1, 10]. However, there is little known about the probable impact of the above factors on the exhibition and the effect of dysphagia. Clayton et al. conducted research to describe the physiological characteristics of dysphagia, its recovery pattern, and its outcomes in 27 ICU patients affected by this disease. They recognized an association between the severity of dysphagia and the length of intubation, MV, ICU LOS, and hospital LOS variables [18].
Further, no studies are reporting the association between cognitive/consciousness factors and the presence of dysphagia in patients due to COVID-19 hospitalized in the ICU. Identifying predictors for dysphagia will provide the means to the appropriate healthcare professionals for careful monitoring and or assessment. By extension, routine and sustained management will provide the basis for developing protocols that can ensure timely and efficient care while mitigating potential serious complications. As a result, the purposes of our quest were to determine the incidence of dysphagia in patients with COVID-19 hospitalized into the ICU and examine the relationship between demographic/clinical factors and cognition/consciousness to develop dysphagia in these patients. Awareness of the incidence and hazard factors can warrant that dysphagic adults are sharply distinguished and assessed to diminish medical and quality-of-life worries [19].
Materials and Methods
Study participants
We prospectively recruited 100 patients admitted to the ICUs of Firoozgar Hospital in Tehran City, Iran between July and October 2022, based on the following inclusion criteria: 1) Admitted patients with positive COVID-19 founded on polymerase Chain reaction test; 2) Age between 18 and 65 years; 3) No history of dysphagia before being affected by COVID-19; 4) No history of neurodegenerative diseases before being affected by COVID-19; and 5) No dysphagia diagnosis at the time of entering to the ICU. Meanwhile, the exclusion criteria were incomplete required information in the patient’s medical record and patients suffering from brain problems due to COVID-19 disease.
We performed the present research on the COVID-19 patients admitted to the ICU and then transferred to the COVID-19 ward of Firoozgar Hospital. All patients hospitalized in the ICU were evaluated for the inclusion criteria if the consent was obtained by the patient or their first-grade relative. In the first hours of the patient’s admission to the ICU, demographic and clinical information, including age, sex, comorbidities, number of vaccination doses, body mass index (BMI), the severity of lung involvement, and level of patient consciousness were extracted from the medical records. All eligible patients underwent the Persian version of the functional oral intake scale (FOIS-P) for swallowing disorder screening, the Rancho Los Amigos scale (RLAS), for patients’ cognitive status by the study of speech and language pathologists with four years of experience. As we aimed to evaluate the incidence of dysphagia, the patient was excluded if there was dysphagia at the time of admission, and patients who had normal swallowing in the initial evaluation were included. The speech and language pathologist monitored the patients daily and completed the FOIS-P questionnaire if there were any symptoms of dysphagia throughout hospitalization in the ICU as well as the hospital ward. Patients who underwent intubation were not evaluated for swallowing due to the distortion of the results of swallowing status. These patients were evaluated 24 h after extubation. At the time of diagnosis of dysphagia, other information, including the presence or absence of intubation and tracheostomy (days), presence or absence of prone positioning (days), and receiving or not receiving sedative (days), were extracted from the medical record.
Study instruments
The FOIS-P
FOIS is a 7-point ordinal measure tool in which levels one to five are defined as dysphagia and levels 6 to 7 are defined as practical swallowing [20]. Crary et al. developed FOIS to measure oral fluid and food consumption in patients with stroke. They described the high validity and reliability of the tool at 90% and 85%, respectively [21]. Bakhtiyari et al. validated the Persian version of this scale for patients with stroke. According to their results, the reliability of the scale was 89%. Also, they described that the scale has high validity and sensitivity in detecting swallowing disorders [22]. FOIS has been used for the evaluation of dysphagia in some other conditions [23-25] as well as COVID-19 [8, 26].
The RLAS
RLAS is an acknowledged medical scale applied to define cognitive and behavioral patterns in patients with brain injury [27]. The scale has 8 levels that the case’s cognitive level is assessed based on a series of behaviors determined at any level. For example, if the case does not display any response to sound, light, touch, or pain, the case will be at the initial level of the RLAS. As the case evolves to higher levels, they display improved cognitive and behavioral positions and move toward more independence [28].
Data analyses
We reported the Mean±SD for the quantitative variables and percentage and frequency for qualitative variables. In addition, we used a binary logistic regression analysis to identify factors that are related to swallowing disorders. All of these were executed using the SPSS software, version 22.
Results
Recruitment rate
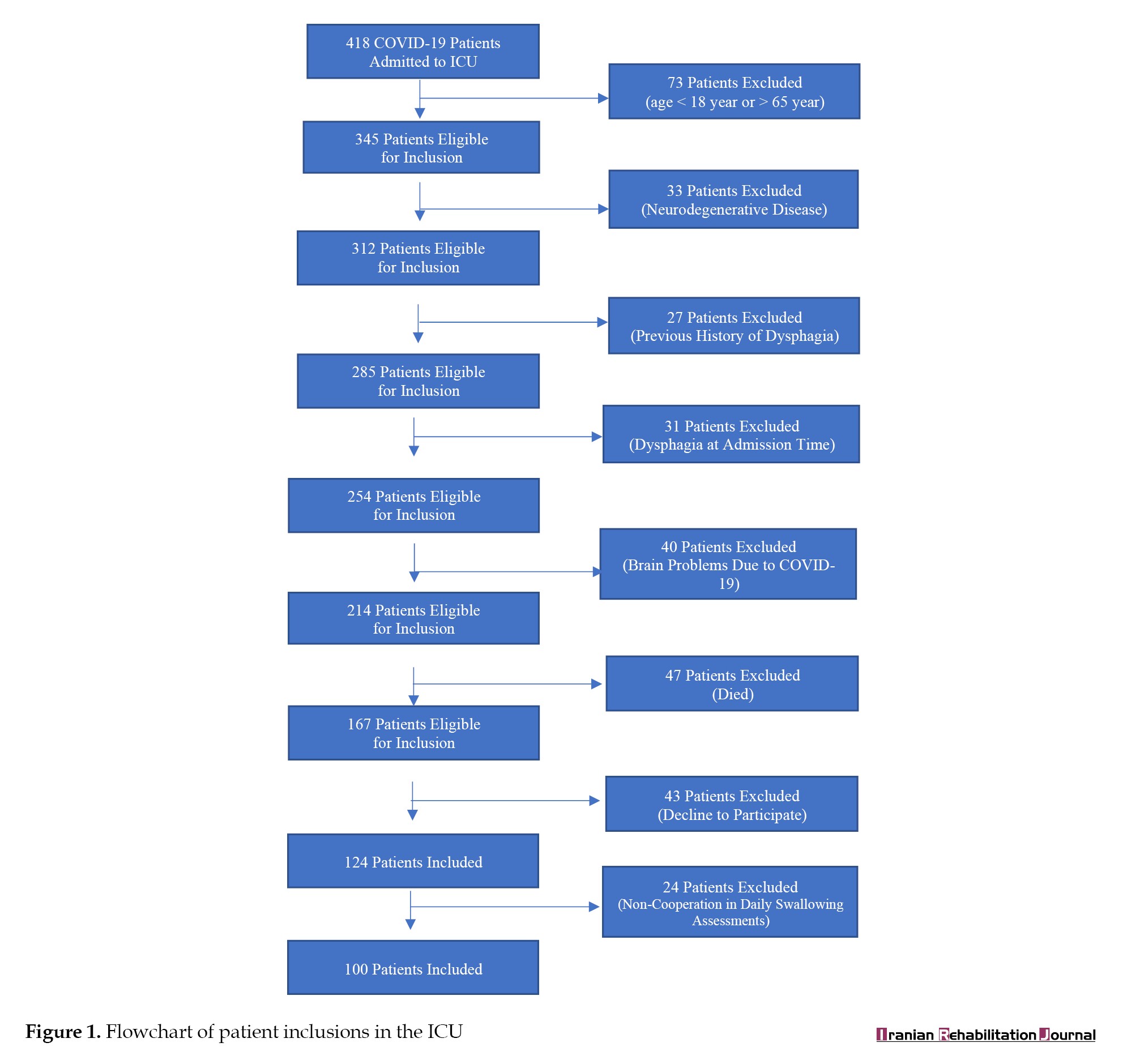
Between July to October 2022, a total of 418 patients affected by COVID-19 were admitted to the ICUs of Firoozgar Hospital. About 251 patients did not meet the inclusion criteria and from the remaining 40%, 43 patients did not fill out the informed consent form and 24 did not cooperate in the daily evaluation of swallowing status (Figure 1). Finally, 100 patients participated in the study with informed consent.
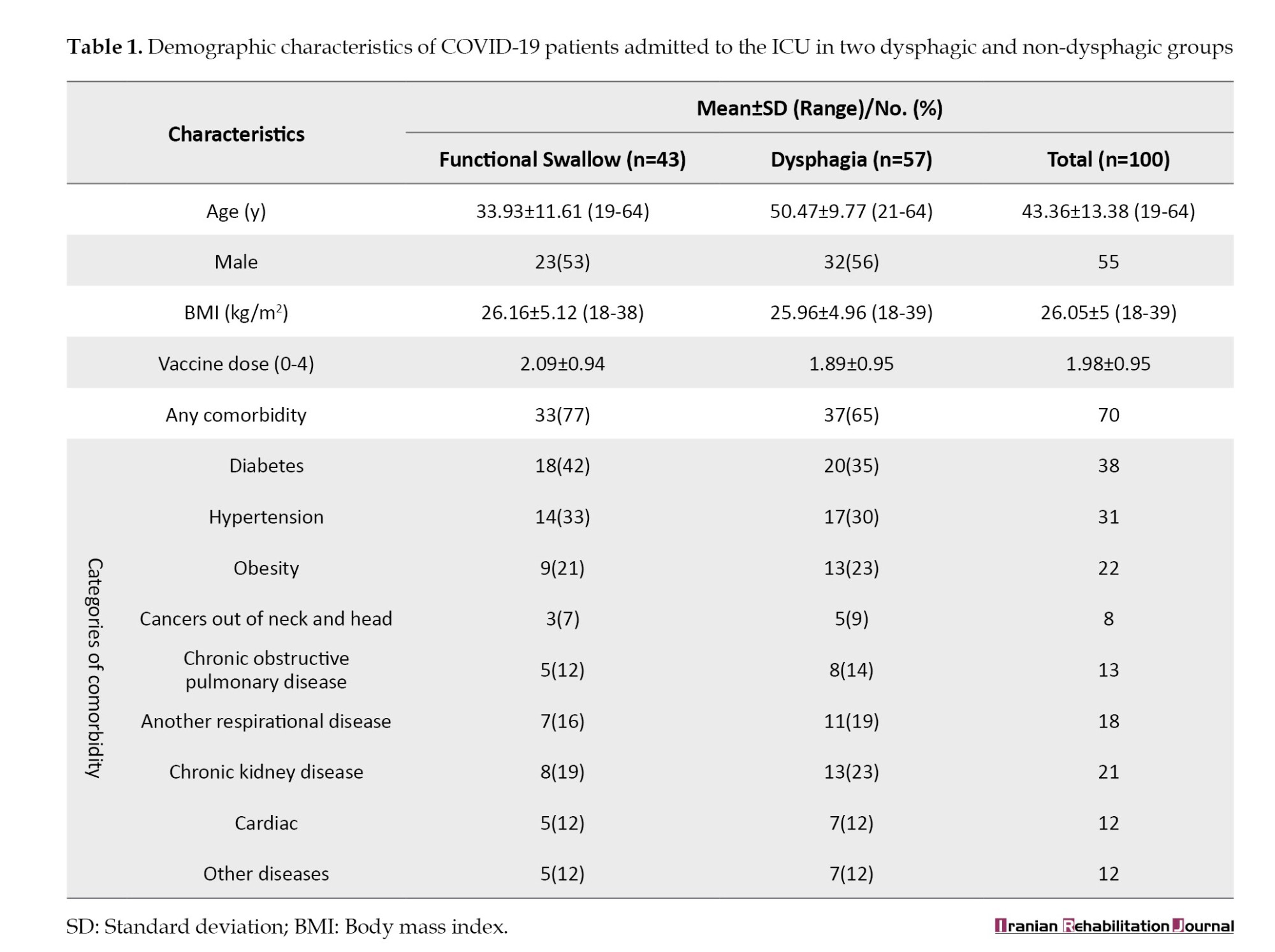
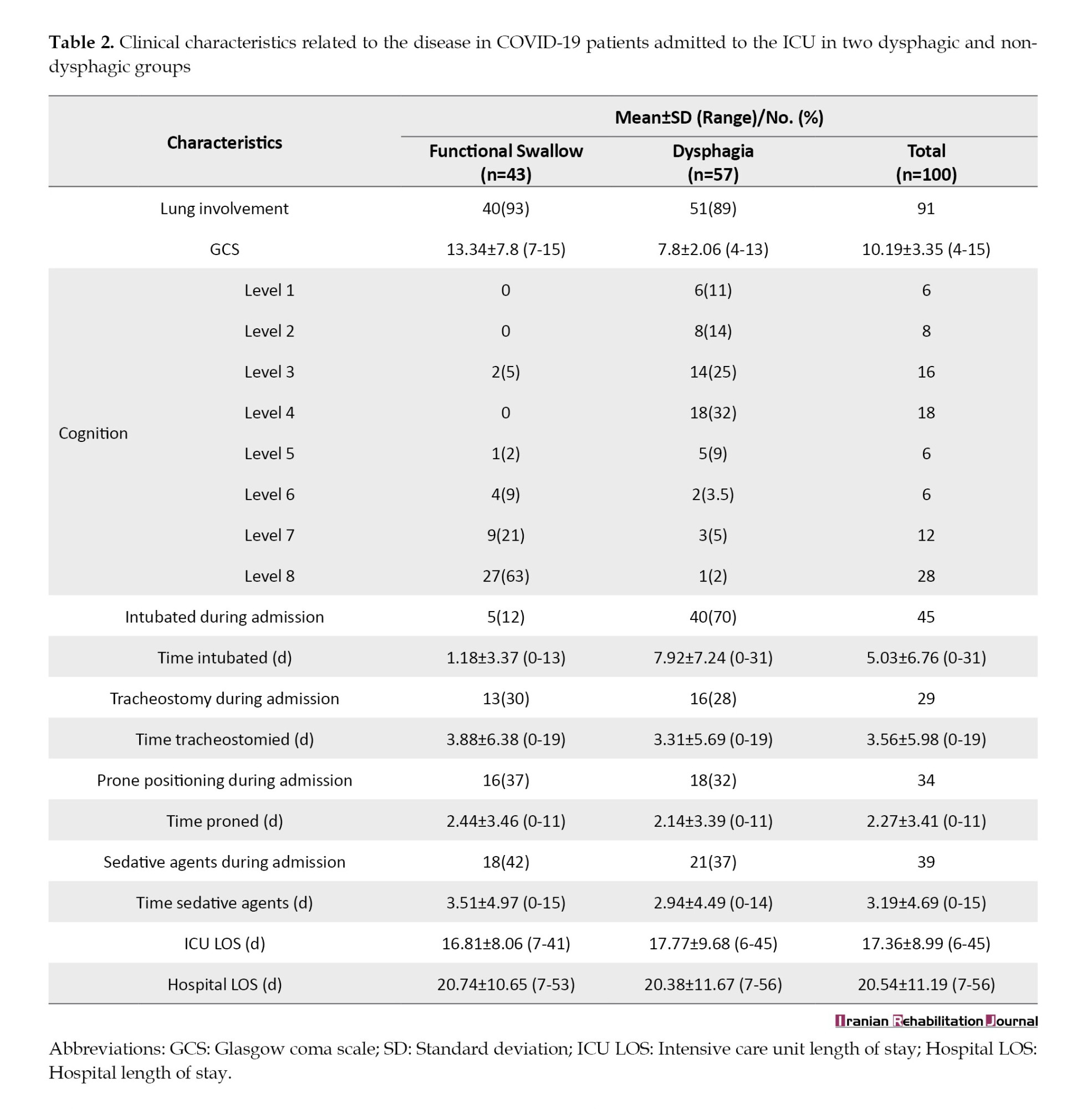
The mean age of included patients was 43.3±13.3 years, with 55% being male. The consciousness of the patients based on the Glasgow coma scale (GCS) varied between 4-15 and an average of 10.19. Out of 100 patients, 9% of the patients did not have any suffering lung involvement but 33% had mild, 34% had moderate, and 24% had severe lung problems. A variety of comorbidities were seen in the patients and 70% of them had at least one comorbidity, with the most common ones being diabetes (38%), hypertension (31%), and obesity (22%). A total of 74% needed invasive MV, of which 60% were intubated and 40% underwent tracheostomy. In addition, 34% of 100 patients were placed in prone positioning during hospitalization. Also, sedatives were prescribed for 39% of the patients. Demographic, medical, and disease-related features of the study sample, like age, gender, comorbidities, MV, prone positioning, sedatives, and severity of lung involvement are presented in Tables 1 and 2.
Incidence of dysphagia
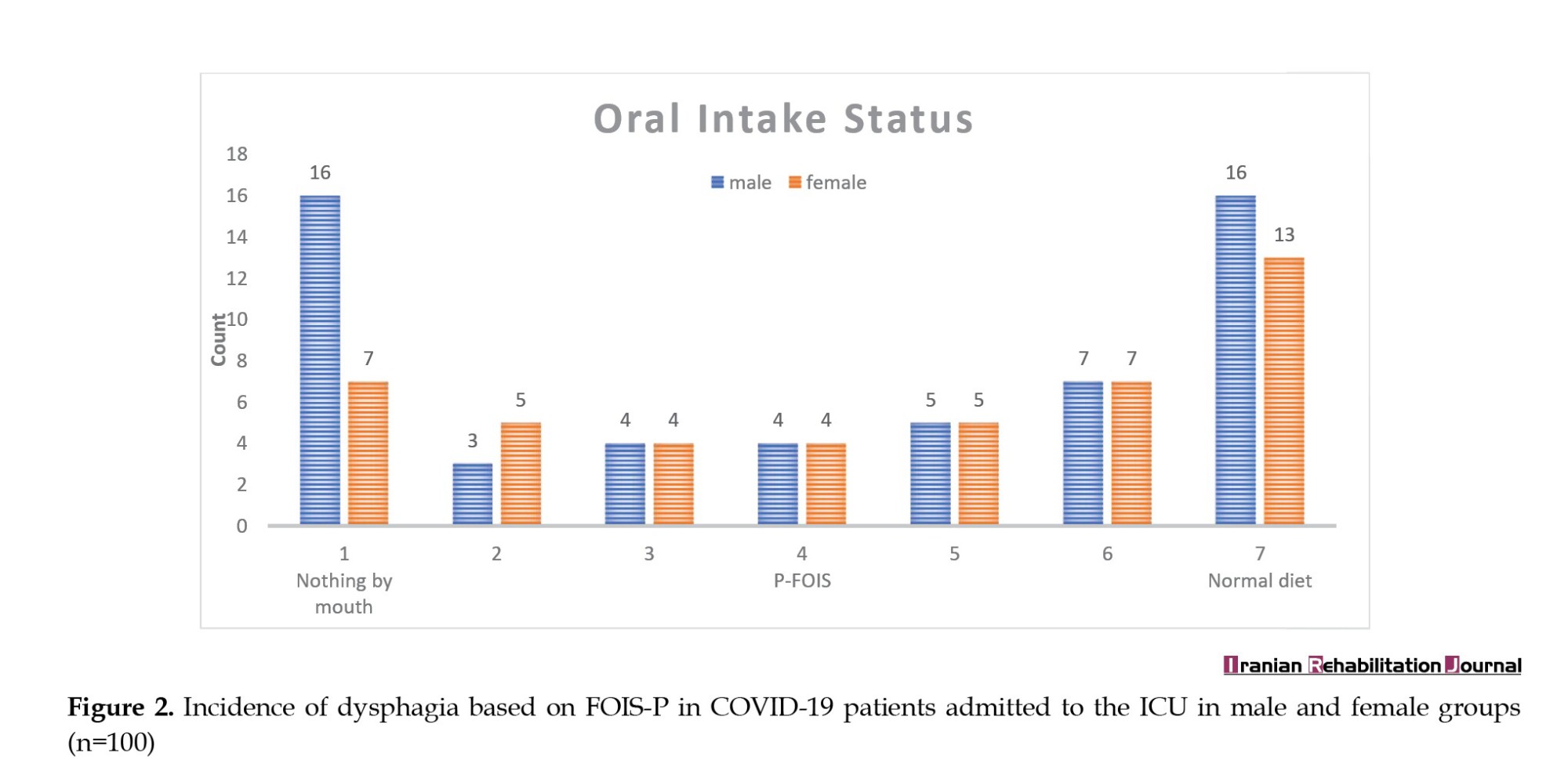
Clinical signs of dysphagia (FOIS-P, items 1-5) were found in 57 of 100 patients. In detail, 43% of patients were on a normal (FOIS-P, item 7), or soft normal diet (FOIS-P, item 6). In contrast, 39% required tube feeding (FOIS-P, item 1–3), and 23% (FOIS-P, item 1) were nil by mouth (Figure 2). Based on the gender breakdown, 56% of the population with dysphagia were men and 44% were women.
Predictor variables of dysphagia
Univariate regression analysis was performed for all variables. Based on the results, age, level of consciousness, presence or absence of intubation, number of days of intubation, as well as cognition had a P<0.001 and they were significantly correlated with the occurrence of dysphagia. In the next step, variables with P<0.2 in the univariate model were arrived into the multivariate model to adjust the effect of confounders. The odds ratio (OR) and P are reported in Table 3.
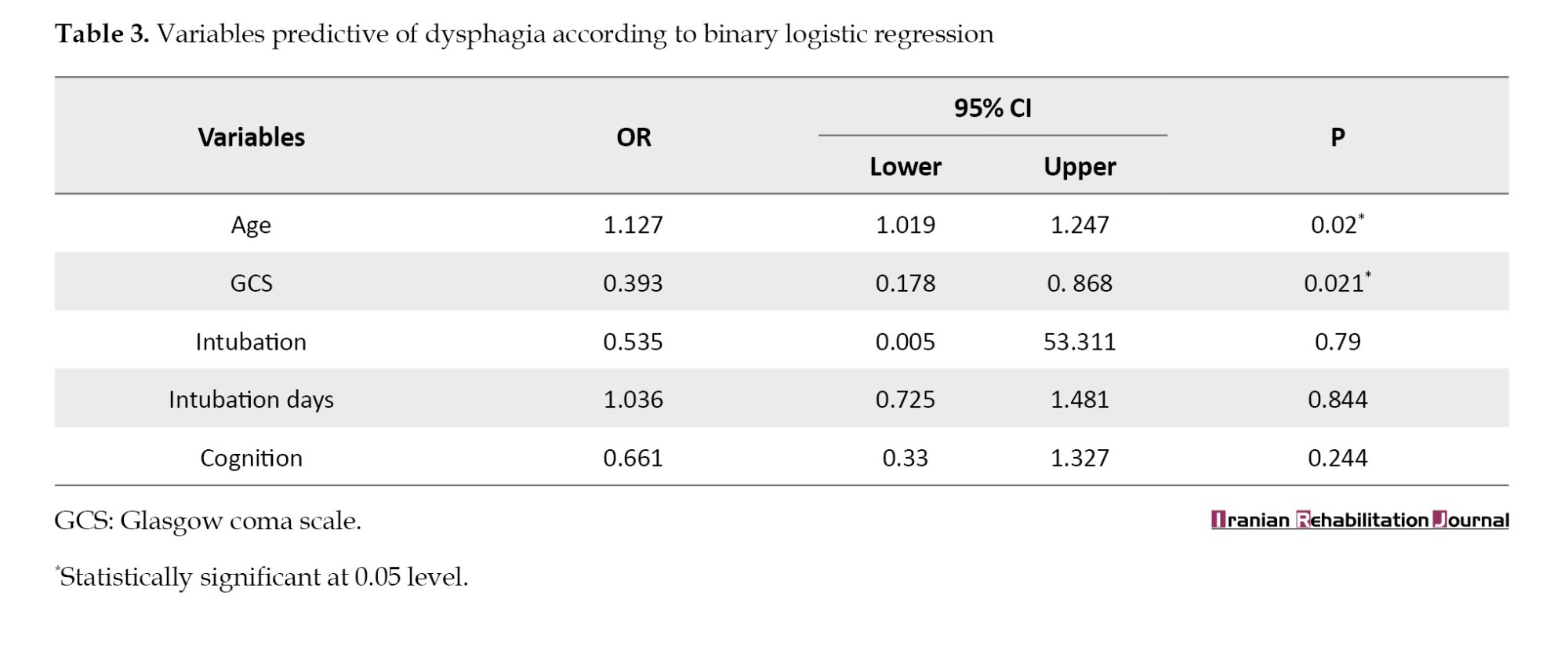
Age (OR=1.127; 95% confidence interval [CI], 1.019%, 1.247%; P=0.02) and the level of consciousness (OR=0.393; 95% CI, 0.178%, 0.868%; P=0.021) are significant predictors of oral consumption status. The results showed that with increasing age, the probability of the occurrence of swallowing disorder is 1.127 higher than normal. Also, with a decrease in consciousness, the probability rate of dysphagia increases up to 40%.
Discussion
According to the author’s information, this is the first prospective cohort study that examined swallowing impairment among COVID-19 patients admitted to ICU in Tehran City, Iran. A high frequency of dysphagia was observed among patients, and clear risk factors related to the occurrence of dysphagia were distinguished that can enable primitive exploration and management of these patients who have swallowing impairment associated with COVID-19.
The COVID-19 infection has been an active pandemic disease. Research on its clinical symptoms is still ongoing [29]. Current study findings demonstrated a high incidence of dysphagia with an estimated 57% (n=57) of patients hospitalized in ICUs who were contaminated with the virus. This finding is in line with the study of Ceruti et al. which was a retrospective study in Switzerland that evaluated swallowing by gagging swallowing screen in 31 patients in the ICU. They reported that the prevalence of swallowing disorders in these patients is 54.8% [16]. Similarly, Martin-Martinez et al. conducted a prospective observational study on 205 patients who were hospitalized in the COVID-19 ward of a therapeutic center located in Spain. The prevalence of dysphagia was 51.7% according to eating assessment tool-10 and volume-viscosity swallowing test [30]. In contrast, based on the results of an observational cohort study conducted by Regan et al. the prevalence of dysphagia after intubation in 100 COVID-19 patients admitted to 11 hospitals across Ireland, was reported to be much higher (86%) [8]. One of the main reasons for this difference between the results of Regan et al.’s study and the current study is that they included 100 patients with COVID-19 disease, all of whom were intubated, and examined the amount of post-extubation dysphagia [8]. While the pathophysiology of post-extubation dysphagia is different and it is a rampant difficulty in COVID-19 patients after ICU admission [30]; however, in the present study, only 45% (45) of patients underwent intubation. In addition, Regan et al. included patients who had stroke, progressive neurodegenerative diseases, dementia, and head and neck cancers before or after affecting by the COVID-19 disease in their study [8]. Stroke or other neurological diseases can disrupt multiple parts of the deglutition neural network and make the patients susceptible to dysphagia [30]. Nevertheless, these patients were excluded from the present study.
One of the causes of the occurrence of dysphagia in this type of patient is respiratory difficulties associated with lung involvement in COVID-19 disease which may lead to loss of swallowing-breathing coordination [31]. Swallowing and breathing have shown a complex relationship and both systems apply common elements in neurological, physiological, structural, and functional fields. Swallowing arises from an exhalation-swallowing-exhalation plan, which leads to the protection of the airway and respiratory system. The lack of this coordination can lead to dehydration, pneumonia, and aspiration, reducing the patient’s life satisfaction, and thus compromising their prognosis [9].
In multivariate regression analysis, we acknowledged that age has been correlated with worse swallowing outcomes. Therefore, as the age increases, the possibility of swallowing disorder in COVID-19 patients is higher. Regan et al. also reported that age is a predictor for dysphagia in COVID-19 patients [8, 19]. Comorbidities amongst the elderly, cachexia, and sarcopenia, which are common syndromes in older patients, can make a deal with swallowing action [19]. Older individuals might have a presbyphagia, that predisposes these people to swallowing difficulties [8]. Dysphagia is detected more frequently in elderly patients due to muscle atrophy, sensorimotor changes, and connective tissue weakening [32].
Furthermore, the level of consciousness at admission was identified as an independent predictor of dysphagia. Therefore, dysphagia was more common in patients with low GCS. In studies that investigated dysphagia in patients with COVID-19, the level of consciousness was not considered. In previous studies, a significant relationship has been found between the level of consciousness and swallowing disorder in patients with stroke [33]. Barer observed that all stroke patients, who did not have an impaired level of consciousness in the initial stage of the disease, had better improvement in the symptoms of the swallowing disorder [33]. Recently, Mélotte et al. conducted a literature review to investigate debated studies that paid attention to the relationship between consciousness and swallowing. They reported that in severe brain injury patients and patients with consciousness disorders, the level of consciousness is associated with dysphagia [34].
Accordingly, it is necessary for all hospitalized COVID-19 patients to be screened for swallowing impairments. If any impairments are detected, to avoid nutritional complications and pneumonia aspiration they also must undergo a deglutition assessment [35]. Mindfulness of the numerous hazard factors may be beneficial in that it can ensure that dysphagic patients are identified sharply and assessed so that the medical and quality of life worries can be reduced [19].
Conclusion
This study showed that the incidence of dysphagia in adult patients infected by COVID-19 hospitalized in the ICUs is high and equal to 57%. Meanwhile, age and level of consciousness were positively linked with dysphagia occurrence in this group of patients. Knowledge of the incidence rate of dysphagia and its hazard factors enables healthcare professionals to accurately monitor and evaluate swallowing symptoms to reduce its clinical and quality of life complications.
Study limitations and future research
In the present study, dysphagia diagnosis was questionnaire-based, without confirmatory instrumental evaluation. Fiberoptic endoscopic evaluation of swallowing and videofluoroscopy have not been available in hospital settings due to concerns regarding the contagious jeopardy of disease. Fiberoptic endoscopic evaluation of swallowing can provide a huge number of practical information like pharyngeal sensation, secretions, residue, and or aspiration. Furthermore, this was a single-center observational study. Future research with multiple treatment centers will be obligatory to approve the introductory data. Finally, we determined the incidence of swallowing disorder during hospitalization and it was focused on in-hospital outcomes. Further studies should also investigate the effects of COVID-19 in three- or six-month follow-ups.
Despite all these limitations, the present study is the first cohort investigating the incidence and related factors affecting dysphagia, specifically the level of consciousness and cognition in COVID-19 patients by daily monitoring the swallowing status of the patients during their hospitalization.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Human Ethics Research Committee of the University of Social Welfare and Rehabilitation Sciences (Code: IR.USWR.REC.1401.066).Informed consent was obtained and the participants were informed. If the patient had not been conscious enough, their first-degree relatives would have signed the consent.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Akbar Banari, Zahra Sadeghi and Akbar Darouie; Investigation: Akbar Banari and Niloofar Masoudian; Formal analysis: Mehdi Noroozi and Akbar Banari; Supervision: Zahra Sadeghi and Akbar Darouie; Methodology, project administration, data curation, and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors of the research sincerely thank the patients and their families for participating in the study. Also, we would like to thank the staff of the COVID-19 ward and ICU of Firoozgar Hospital who supported this paper in the data collection phase.
References
Coronavirus disease 2019 (COVID-19) which is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) first appeared in December 2019 in China. As the disease was highly contagious, it affected the world and started a pandemic [1]. The symptoms of this respiratory disease are diverse. Sometimes the disease can be latent without symptoms and sometimes it can threaten a person’s life, resulting in hospitalization and admission to intensive care units (ICUs) [2-4].
Common complications of hospitalized COVID-19 patients include sepsis, shock, acute respiratory distress syndrome, acute kidney failure, neurologic conditions, acute respiratory failure, acute ischemic heart disease, and venous thromboembolism [5]. COVID-19 mostly disturbs the respiratory system and can lead to acute respiratory distress syndrome [6]. These patients may require invasive respiratory support devices, such as nasogastric tube or mechanical ventilation (MV) [7]. Administering respiratory support devices may increase the risk of dysphagia in these patients [8]. Furthermore, dysphagia in COVID-19 patients results from incoordination between swallowing and breathing [9]. Medications prescribed for COVID-19, including corticosteroids which develop myopathy as a side effect, hydroxychloroquine which raises corticosteroid myopathy, and sedation and relaxation for a long period can cause swallowing impairment [10]. Dysphagia is associated with severe and multifaceted poor outcomes, such as malnutrition, aspiration pneumonia, dehydration, extended hospitalization, and raised mortality rates [9, 11]. Consequently, continued monitoring of patients for risk of dysphagia is warranted. Establishing the predictor factors of developing dysphagia will alert medical staff for careful monitoring and can contribute to the evolvement of management protocols for efficient and well-timed care that prevents its severe outcomes [8].
Swallowing is a complicated cognitive-sensorimotor process regulated by several brain areas spreading from higher cortical regions to the central pattern generator in the lateral medulla due to closely coordinated and sequential voluntary and involuntary behaviors for the anticipatory, oral preparatory, oral transit, and pharyngeal phases of the swallow [12]. Magnetoencephalography studies demonstrating the temporal features of cortical activation during intentional swallowing display the activity of the left primary sensorimotor cerebral cortex during voluntary swallowing. Further, other studies have specified that the cingulate cortex, the insula, and the inferior frontal gyrus are activated by each swallow [13]. Swallowing is a reflexive reaction and a stream of complex cognitive processes involving timely initiation of the swallowing reflex and maintaining safe swallowing [14].
The prevalence of swallowing issues in this group of patients has shown different figures, from 7% to 55% [15, 16]. Bordejé Laguna et al. conducted a study investigating the dysphagia incidence in 93 patients with severe pneumonia due to SARS-CoV-2, who required MV. The authors used the modified viscosity volume swallowing test to evaluate the swallowing status. The findings of this study showed that the incidence of dysphagia in these patients is 26.9% [10]. In a study by Rouhani et al. an eating assessment tool questionnaire was applied to a group of 41 patients who ought to have tracheostomy to treat respiratory problems caused by COVID-19. The study demonstrated that 30% of the patients had abnormal swallowing [17].
Different researchers have tried to understand the relationship between swallowing disorders and factors, for instance, demographic variables, including gender, age, comorbidities, and clinical factors, including MV variables, prone positioning, sedative agents, ICU, and hospital long of stay (LOS) [1, 10]. However, there is little known about the probable impact of the above factors on the exhibition and the effect of dysphagia. Clayton et al. conducted research to describe the physiological characteristics of dysphagia, its recovery pattern, and its outcomes in 27 ICU patients affected by this disease. They recognized an association between the severity of dysphagia and the length of intubation, MV, ICU LOS, and hospital LOS variables [18].
Further, no studies are reporting the association between cognitive/consciousness factors and the presence of dysphagia in patients due to COVID-19 hospitalized in the ICU. Identifying predictors for dysphagia will provide the means to the appropriate healthcare professionals for careful monitoring and or assessment. By extension, routine and sustained management will provide the basis for developing protocols that can ensure timely and efficient care while mitigating potential serious complications. As a result, the purposes of our quest were to determine the incidence of dysphagia in patients with COVID-19 hospitalized into the ICU and examine the relationship between demographic/clinical factors and cognition/consciousness to develop dysphagia in these patients. Awareness of the incidence and hazard factors can warrant that dysphagic adults are sharply distinguished and assessed to diminish medical and quality-of-life worries [19].
Materials and Methods
Study participants
We prospectively recruited 100 patients admitted to the ICUs of Firoozgar Hospital in Tehran City, Iran between July and October 2022, based on the following inclusion criteria: 1) Admitted patients with positive COVID-19 founded on polymerase Chain reaction test; 2) Age between 18 and 65 years; 3) No history of dysphagia before being affected by COVID-19; 4) No history of neurodegenerative diseases before being affected by COVID-19; and 5) No dysphagia diagnosis at the time of entering to the ICU. Meanwhile, the exclusion criteria were incomplete required information in the patient’s medical record and patients suffering from brain problems due to COVID-19 disease.
We performed the present research on the COVID-19 patients admitted to the ICU and then transferred to the COVID-19 ward of Firoozgar Hospital. All patients hospitalized in the ICU were evaluated for the inclusion criteria if the consent was obtained by the patient or their first-grade relative. In the first hours of the patient’s admission to the ICU, demographic and clinical information, including age, sex, comorbidities, number of vaccination doses, body mass index (BMI), the severity of lung involvement, and level of patient consciousness were extracted from the medical records. All eligible patients underwent the Persian version of the functional oral intake scale (FOIS-P) for swallowing disorder screening, the Rancho Los Amigos scale (RLAS), for patients’ cognitive status by the study of speech and language pathologists with four years of experience. As we aimed to evaluate the incidence of dysphagia, the patient was excluded if there was dysphagia at the time of admission, and patients who had normal swallowing in the initial evaluation were included. The speech and language pathologist monitored the patients daily and completed the FOIS-P questionnaire if there were any symptoms of dysphagia throughout hospitalization in the ICU as well as the hospital ward. Patients who underwent intubation were not evaluated for swallowing due to the distortion of the results of swallowing status. These patients were evaluated 24 h after extubation. At the time of diagnosis of dysphagia, other information, including the presence or absence of intubation and tracheostomy (days), presence or absence of prone positioning (days), and receiving or not receiving sedative (days), were extracted from the medical record.
Study instruments
The FOIS-P
FOIS is a 7-point ordinal measure tool in which levels one to five are defined as dysphagia and levels 6 to 7 are defined as practical swallowing [20]. Crary et al. developed FOIS to measure oral fluid and food consumption in patients with stroke. They described the high validity and reliability of the tool at 90% and 85%, respectively [21]. Bakhtiyari et al. validated the Persian version of this scale for patients with stroke. According to their results, the reliability of the scale was 89%. Also, they described that the scale has high validity and sensitivity in detecting swallowing disorders [22]. FOIS has been used for the evaluation of dysphagia in some other conditions [23-25] as well as COVID-19 [8, 26].
The RLAS
RLAS is an acknowledged medical scale applied to define cognitive and behavioral patterns in patients with brain injury [27]. The scale has 8 levels that the case’s cognitive level is assessed based on a series of behaviors determined at any level. For example, if the case does not display any response to sound, light, touch, or pain, the case will be at the initial level of the RLAS. As the case evolves to higher levels, they display improved cognitive and behavioral positions and move toward more independence [28].
Data analyses
We reported the Mean±SD for the quantitative variables and percentage and frequency for qualitative variables. In addition, we used a binary logistic regression analysis to identify factors that are related to swallowing disorders. All of these were executed using the SPSS software, version 22.
Results
Recruitment rate
Between July to October 2022, a total of 418 patients affected by COVID-19 were admitted to the ICUs of Firoozgar Hospital. About 251 patients did not meet the inclusion criteria and from the remaining 40%, 43 patients did not fill out the informed consent form and 24 did not cooperate in the daily evaluation of swallowing status (Figure 1). Finally, 100 patients participated in the study with informed consent.

The mean age of included patients was 43.3±13.3 years, with 55% being male. The consciousness of the patients based on the Glasgow coma scale (GCS) varied between 4-15 and an average of 10.19. Out of 100 patients, 9% of the patients did not have any suffering lung involvement but 33% had mild, 34% had moderate, and 24% had severe lung problems. A variety of comorbidities were seen in the patients and 70% of them had at least one comorbidity, with the most common ones being diabetes (38%), hypertension (31%), and obesity (22%). A total of 74% needed invasive MV, of which 60% were intubated and 40% underwent tracheostomy. In addition, 34% of 100 patients were placed in prone positioning during hospitalization. Also, sedatives were prescribed for 39% of the patients. Demographic, medical, and disease-related features of the study sample, like age, gender, comorbidities, MV, prone positioning, sedatives, and severity of lung involvement are presented in Tables 1 and 2.


Incidence of dysphagia
Clinical signs of dysphagia (FOIS-P, items 1-5) were found in 57 of 100 patients. In detail, 43% of patients were on a normal (FOIS-P, item 7), or soft normal diet (FOIS-P, item 6). In contrast, 39% required tube feeding (FOIS-P, item 1–3), and 23% (FOIS-P, item 1) were nil by mouth (Figure 2). Based on the gender breakdown, 56% of the population with dysphagia were men and 44% were women.

Predictor variables of dysphagia
Univariate regression analysis was performed for all variables. Based on the results, age, level of consciousness, presence or absence of intubation, number of days of intubation, as well as cognition had a P<0.001 and they were significantly correlated with the occurrence of dysphagia. In the next step, variables with P<0.2 in the univariate model were arrived into the multivariate model to adjust the effect of confounders. The odds ratio (OR) and P are reported in Table 3.

Age (OR=1.127; 95% confidence interval [CI], 1.019%, 1.247%; P=0.02) and the level of consciousness (OR=0.393; 95% CI, 0.178%, 0.868%; P=0.021) are significant predictors of oral consumption status. The results showed that with increasing age, the probability of the occurrence of swallowing disorder is 1.127 higher than normal. Also, with a decrease in consciousness, the probability rate of dysphagia increases up to 40%.
Discussion
According to the author’s information, this is the first prospective cohort study that examined swallowing impairment among COVID-19 patients admitted to ICU in Tehran City, Iran. A high frequency of dysphagia was observed among patients, and clear risk factors related to the occurrence of dysphagia were distinguished that can enable primitive exploration and management of these patients who have swallowing impairment associated with COVID-19.
The COVID-19 infection has been an active pandemic disease. Research on its clinical symptoms is still ongoing [29]. Current study findings demonstrated a high incidence of dysphagia with an estimated 57% (n=57) of patients hospitalized in ICUs who were contaminated with the virus. This finding is in line with the study of Ceruti et al. which was a retrospective study in Switzerland that evaluated swallowing by gagging swallowing screen in 31 patients in the ICU. They reported that the prevalence of swallowing disorders in these patients is 54.8% [16]. Similarly, Martin-Martinez et al. conducted a prospective observational study on 205 patients who were hospitalized in the COVID-19 ward of a therapeutic center located in Spain. The prevalence of dysphagia was 51.7% according to eating assessment tool-10 and volume-viscosity swallowing test [30]. In contrast, based on the results of an observational cohort study conducted by Regan et al. the prevalence of dysphagia after intubation in 100 COVID-19 patients admitted to 11 hospitals across Ireland, was reported to be much higher (86%) [8]. One of the main reasons for this difference between the results of Regan et al.’s study and the current study is that they included 100 patients with COVID-19 disease, all of whom were intubated, and examined the amount of post-extubation dysphagia [8]. While the pathophysiology of post-extubation dysphagia is different and it is a rampant difficulty in COVID-19 patients after ICU admission [30]; however, in the present study, only 45% (45) of patients underwent intubation. In addition, Regan et al. included patients who had stroke, progressive neurodegenerative diseases, dementia, and head and neck cancers before or after affecting by the COVID-19 disease in their study [8]. Stroke or other neurological diseases can disrupt multiple parts of the deglutition neural network and make the patients susceptible to dysphagia [30]. Nevertheless, these patients were excluded from the present study.
One of the causes of the occurrence of dysphagia in this type of patient is respiratory difficulties associated with lung involvement in COVID-19 disease which may lead to loss of swallowing-breathing coordination [31]. Swallowing and breathing have shown a complex relationship and both systems apply common elements in neurological, physiological, structural, and functional fields. Swallowing arises from an exhalation-swallowing-exhalation plan, which leads to the protection of the airway and respiratory system. The lack of this coordination can lead to dehydration, pneumonia, and aspiration, reducing the patient’s life satisfaction, and thus compromising their prognosis [9].
In multivariate regression analysis, we acknowledged that age has been correlated with worse swallowing outcomes. Therefore, as the age increases, the possibility of swallowing disorder in COVID-19 patients is higher. Regan et al. also reported that age is a predictor for dysphagia in COVID-19 patients [8, 19]. Comorbidities amongst the elderly, cachexia, and sarcopenia, which are common syndromes in older patients, can make a deal with swallowing action [19]. Older individuals might have a presbyphagia, that predisposes these people to swallowing difficulties [8]. Dysphagia is detected more frequently in elderly patients due to muscle atrophy, sensorimotor changes, and connective tissue weakening [32].
Furthermore, the level of consciousness at admission was identified as an independent predictor of dysphagia. Therefore, dysphagia was more common in patients with low GCS. In studies that investigated dysphagia in patients with COVID-19, the level of consciousness was not considered. In previous studies, a significant relationship has been found between the level of consciousness and swallowing disorder in patients with stroke [33]. Barer observed that all stroke patients, who did not have an impaired level of consciousness in the initial stage of the disease, had better improvement in the symptoms of the swallowing disorder [33]. Recently, Mélotte et al. conducted a literature review to investigate debated studies that paid attention to the relationship between consciousness and swallowing. They reported that in severe brain injury patients and patients with consciousness disorders, the level of consciousness is associated with dysphagia [34].
Accordingly, it is necessary for all hospitalized COVID-19 patients to be screened for swallowing impairments. If any impairments are detected, to avoid nutritional complications and pneumonia aspiration they also must undergo a deglutition assessment [35]. Mindfulness of the numerous hazard factors may be beneficial in that it can ensure that dysphagic patients are identified sharply and assessed so that the medical and quality of life worries can be reduced [19].
Conclusion
This study showed that the incidence of dysphagia in adult patients infected by COVID-19 hospitalized in the ICUs is high and equal to 57%. Meanwhile, age and level of consciousness were positively linked with dysphagia occurrence in this group of patients. Knowledge of the incidence rate of dysphagia and its hazard factors enables healthcare professionals to accurately monitor and evaluate swallowing symptoms to reduce its clinical and quality of life complications.
Study limitations and future research
In the present study, dysphagia diagnosis was questionnaire-based, without confirmatory instrumental evaluation. Fiberoptic endoscopic evaluation of swallowing and videofluoroscopy have not been available in hospital settings due to concerns regarding the contagious jeopardy of disease. Fiberoptic endoscopic evaluation of swallowing can provide a huge number of practical information like pharyngeal sensation, secretions, residue, and or aspiration. Furthermore, this was a single-center observational study. Future research with multiple treatment centers will be obligatory to approve the introductory data. Finally, we determined the incidence of swallowing disorder during hospitalization and it was focused on in-hospital outcomes. Further studies should also investigate the effects of COVID-19 in three- or six-month follow-ups.
Despite all these limitations, the present study is the first cohort investigating the incidence and related factors affecting dysphagia, specifically the level of consciousness and cognition in COVID-19 patients by daily monitoring the swallowing status of the patients during their hospitalization.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Human Ethics Research Committee of the University of Social Welfare and Rehabilitation Sciences (Code: IR.USWR.REC.1401.066).Informed consent was obtained and the participants were informed. If the patient had not been conscious enough, their first-degree relatives would have signed the consent.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: Akbar Banari, Zahra Sadeghi and Akbar Darouie; Investigation: Akbar Banari and Niloofar Masoudian; Formal analysis: Mehdi Noroozi and Akbar Banari; Supervision: Zahra Sadeghi and Akbar Darouie; Methodology, project administration, data curation, and writing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors of the research sincerely thank the patients and their families for participating in the study. Also, we would like to thank the staff of the COVID-19 ward and ICU of Firoozgar Hospital who supported this paper in the data collection phase.
References
- Yılmaz D, Mengi T, Sarı S. Post-extubation dysphagia and COVID-2019. Türk Nöroloji Dergisi. 2021; 27(Suppl 1):21-5. [DOI:10.4274/tnd.2021.13360]
- Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Network Open. 2020; 3(12):e2029058. [DOI:10.1001/jamanetworkopen.2020.29058] [PMID] [PMCID]
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. The Cochrane Database of Systematic Reviews. 2022; 5(5):CD013665. [DOI:10.1002/14651858.CD013665.pub3] [PMID] [PMCID]
- Hesni E, Sayad B, Khosravi Shadmani F, Najafi F, Khodarahmi R, Rahimi Z, et al. Demographics, clinical characteristics, and outcomes of 27,256 hospitalized COVID-19 patients in Kermanshah Province, Iran: A retrospective one-year cohort study. BMc Infectious Diseases. 2022; 22(1):319. [DOI:10.1186/s12879-022-07312-7] [PMID] [PMCID]
- Abramoff BA, Dillingham TR, Caldera FE, Ritchie MD, Pezzin LE. Inpatient rehabilitation outcomes after severe COVID-19 infections: A retrospective cohort study. American Journal of Physical Medicine & Rehabilitation. 2021; 100(12):1109-14. [DOI:10.1097/PHM.0000000000001885] [PMID] [PMCID]
- Li X, Ma X. Acute respiratory failure in COVID-19: Is it "typical" ARDS? Critical Care. 2020; 24(1):198. [DOI:10.1186/s13054-020-02911-9] [PMID] [PMCID]
- Frajkova Z, Tedla M, Tedlova E, Suchankova M, Geneid A. Postintubation dysphagia during COVID-19 outbreak-contemporary review. Dysphagia. 2020; 35(4):549-57. [DOI:10.1007/s00455-020-10139-6] [PMID] [PMCID]
- Regan J, Walshe M, Lavan S, Horan E, Gillivan Murphy P, Healy A, et al. Post-extubation dysphagia and dysphonia amongst adults with COVID-19 in the republic of Ireland: A prospective multi-site observational cohort study. Clinical Otolaryngology. 2021; 46(6):1290-9. [DOI:10.1111/coa.13832] [PMID] [PMCID]
- Mohan R, Mohapatra B. Shedding light on dysphagia associated with COVID-19: The what and why. OTO Open. 2020; 4(2):2473974X20934770. [DOI:10.1177/2473974X20934770] [PMID] [PMCID]
- Bordejé Laguna L, Marcos-Neira P, de Lagrán Zurbano IM, Marco EM, Guisasola CP, Viñas Soria CD, et al. Dysphagia and mechanical ventilation in SARS-COV-2 pneumonia: It's real. Clinical Nutrition. 2022; 41(12):2927-33. [DOI:10.1016/j.clnu.2021.11.018] [PMID] [PMCID]
- Sadeghi Z, Ghoreishi ZS, Flowers H, Mohammadkhani P, Ashtari F, Noroozi M. Depression, anxiety, and stress relative to swallowing impairment in persons with multiple sclerosis. Dysphagia. 2021; 36(5):902-9. [DOI:10.1007/s00455-020-10207-x] [PMID]
- Jean A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiological Reviews. 2001; 81(2):929-69. [DOI:10.1152/physrev.2001.81.2.929] [PMID]
- Miller AJ. The neurobiology of swallowing and dysphagia. Developmental Disabilities Research Reviews 2008; 14(2):77-86. [DOI:10.1002/ddrr.12] [PMID]
- Ebrahimian Dehaghani S, Yadegari F, Asgari A, Bagheri Z. The mediator effect of cognition on the relationship between brain lesion location and dysphagia in patients with stroke: Applying a structural equation model. Journal of Oral Rehabilitation. 2019; 46(1):33-39. [DOI:10.1111/joor.12722] [PMID]
- Marchese MR, Ausili Cefaro c, mari g, proietti i, carfì a, tosato m, et al. oropharyngeal dysphagia after hospitalization for COVID-19 disease: Our screening results. Dysphagia. 2022; 37(2):447-53. [DOI:10.1007/s00455-021-10325-0] [PMID] [PMCID]
- Ceruti S, Glotta A, Galli A, Biggiogero M, Bona G, Mauri R, et al. Dysphagic disorder in a cohort of COVID-19 patients: Evaluation and evolution. Annals of Medicine and Surgery. 2021; 69:102837. [DOI:10.1016/j.amsu.2021.102837] [PMID] [PMCID]
- Rouhani MJ, Clunie G, Thong G, Lovell L, Roe J, Ashcroft M, et al. A prospective study of voice, swallow, and airway outcomes following tracheostomy for COVID‐19. Laryngoscope. 2021; 131(6):E1918-25. [DOI:10.1002/lary.29346]
- Clayton NA, Walker E, Freeman-Sanderson A. Clinical profile and recovery pattern of dysphagia in the COVID-19 patient: A prospective observational cohort within NSW. Australian Critical Care. 2023; 36(2):262-8. [DOI:10.1016/j.aucc.2022.01.001] [PMID] [PMCID]
- Regan J, Walshe M, Lavan S, Horan E, Murphy PG, Healy A, et al. Dysphagia, dysphonia, and dysarthria outcomes among adults hospitalized with COVID-19 across Ireland. The Laryngoscope. 2022; 132(6):1251-9. [DOI:10.1002/lary.29900] [PMID] [PMCID]
- Lindh MG, Mattsson G, Koyi H, Johansson MB, Razmi R, Palm A. Swallowing function in COVID-19 patients after invasive mechanical ventilation. Archives of Rehabilitation Research and Clinical Translation. 2022; 4(1):100177. [DOI:10.1016/j.arrct.2021.100177] [PMID] [PMCID]
- Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Archives of Physical Medicine and Rehabilitation. 2005; 86(8):1516-20. [DOI:10.1016/j.apmr.2004.11.049] [PMID]
- Bakhtiyari J, Tohidast SA, Mansuri B, Azimi H, Ebadi A. The Persian version of the functional oral intake scale (FOIS-P): A validation study on stroke patients with dysphagia. Logopedics, Phoniatrics, Vocology. 2022; 47(2):133-8. [DOI:10.1080/14015439.2021.1896778] [PMID]
- Heijnen BJ, Speyer R, Baijens LW, Bogaardt HC. Neuromuscular electrical stimulation versus traditional therapy in patients with Parkinson's disease and oropharyngeal dysphagia: Effects on quality of life. Dysphagia. 2012; 27(3):336-45. [DOI:10.1007/s00455-011-9371-z] [PMID] [PMCID]
- Bhatt AD, Goodwin N, Cash E, Bhatt G, Silverman CL, Spanos WJ, et al. Impact of transcutaneous neuromuscular electrical stimulation on dysphagia in patients with head and neck cancer treated with definitive chemoradiation. Head & Neck. 2015; 37(7):1051-6. [DOI:10.1002/hed.23708] [PMID]
- Hansen TS, Engberg AW, Larsen K. Functional oral intake and time to reach unrestricted dieting for patients with traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2008; 89(8):1556-62. [DOI:10.1016/j.apmr.2007.11.063] [PMID]
- Archer SK, Iezzi CM, Gilpin L. Swallowing and voice outcomes in patients hospitalized with COVID-19: An observational cohort study. Archives of Physical Medicine and Rehabilitation. 2021; 102(6):1084-90. [DOI:10.1016/j.apmr.2021.01.063] [PMID] [PMCID]
- Hagen C, Malkmus D, Durham P, Bowman K. Levels of cognitive functioning. Downey, CA: Rancho Los Amigos Hospital. 1972; 6. [Link]
- Lin K, Wroten M. Ranchos los amigos. Treasure Island: StatPearls; 2017. [Link]
- Bennett JE, Blaser MJ, Dolin R. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. Philadelphia: Elsevier Health Sciences; 2014. [Link]
- Martin-Martinez A, Ortega O, Viñas P, Arreola V, Nascimento W, Costa A, et al. COVID-19 is associated with oropharyngeal dysphagia and malnutrition in hospitalized patients during the spring 2020 wave of the pandemic. Clinical Nutrition. 2022; 41(12):2996-3006. [DOI:10.1016/j.clnu.2021.06.010] [PMID] [PMCID]
- Banari A, Aghaz A, Shahriyari A, Fakhimi F, Khoshgoftar M. The Prevalence of Dysphagia in Patients with Covid-19: A systematic review and meta-analysis. International Journal of Health & Medical Research. 2023; 2(7):172-81. [DOI:10.58806/ijhmr.2023.v2i7n03]
- Sura L, Madhavan A, Carnaby G, Crary MA. Dysphagia in the elderly: Management and nutritional considerations. Clinical Interventions in Aging. 2012; 287-98.[DOI:10.2147/CIA.S23404] [PMID] [PMCID]
- Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. Journal of Neurology, Neurosurgery, and Psychiatry. 1989; 52(2):236-41. [DOI:10.1136/jnnp.52.2.236] [PMID] [PMCID]
- Mélotte E, Maudoux A, Panda R, Kaux JF, Lagier A, Herr R, et al. Links Between swallowing and consciousness: A narrative review. Dysphagia. 2023; 38(1):42-64. [DOI:10.1007/s00455-022-10452-2] [PMID]
- Ramos A, Joaquin C, Ros M, Martin M, Cachero M, Sospedra M, et al. Impact of COVID-19 on nutritional status during the first wave of the pandemic. Clinical Nutrition. 2021. [DOI:10.1016/j.clnu.2021.05.001] [PMID] [PMCID]
Article type: Original Research Articles |
Subject:
Speech therapy
Received: 2023/01/11 | Accepted: 2023/05/29 | Published: 2024/06/1
Received: 2023/01/11 | Accepted: 2023/05/29 | Published: 2024/06/1
Send email to the article author





