Volume 22, Issue 2 (June 2024)
Iranian Rehabilitation Journal 2024, 22(2): 345-352 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Bazipoor P, Yalfani A, Karimi M. Evaluation of Chest Wall Motions by Optoelectronic Plethysmography in Scoliosis Patient: A Case Study. Iranian Rehabilitation Journal 2024; 22 (2) :345-352
URL: http://irj.uswr.ac.ir/article-1-1911-en.html
URL: http://irj.uswr.ac.ir/article-1-1911-en.html
1- Department of Exercise Rehabilitation, Faculty of Sport Sciences, Bu- Ali Sina University, Hamedan, Iran.
2- Department of Orthotics and Prosthetics, Faculty of Rehabilitation Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Department of Orthotics and Prosthetics, Faculty of Rehabilitation Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 604 kb]
(935 Downloads)
| Abstract (HTML) (2265 Views)
Full-Text: (602 Views)
Introduction
Scoliosis is a three-dimensional spine deformity characterized by lateral deviation from the normal vertical direction [1, 2]. Scoliosis may occur at any age, without a known cause (idiopathic) or with a known cause [2]. Thoracic spine involvement alone or with the lumbar spine is the primary cause of respiratory or cardiovascular problems [3]. Based on the studies, scoliosis affects respiratory function, including respiratory volumes, chest movements, respiratory muscle strength, respiratory patterns, and respiratory system function during exercise and sleep [2, 4-6].
Scoliosis can cause respiratory disturbances and asymmetry in the chest wall on concave and convex sides. Trunk deformity limits chest mobility, which reduces breathing volume [4].
Regarding the complexity of the relationship among the spine, sternum, and ribs, the displacement and rotation of vertebrae in scoliosis affect the shape of the chest and cause convexity and concavity. Lung inflation will be asymmetrical because the transverse and anteroposterior dimensions of either side of the thorax are substantially different. Furthermore, since the ribs are moving, the chest cavity cannot expand as much as it would otherwise [7].
Various methods can be applied to assess breathing. In particular, spirometry and measuring ventilation gases using a spirometer for measuring respiratory volume are commonly employed. Considering the limitations of these methods, such as using masks and nasal clips and the limitations of use in various conditions, the optoelectronic plethysmography (OEP) method was described as a simple and non-invasive method to measure chest motions and to estimate respiratory volumes [7]. In a study performed by Kotani on chest analysis and diaphragm movements via breathing dynamic magnetic resonance imaging in idiopathic scoliosis patients, it was concluded that chest movements were significantly limited. However, diaphragm movement was normal, and there were correlations between respiratory movements and pulmonary functional tests [5].
In a study conducted by Jones that used the helium dilution closed-circuit technique and the belt restriction method on the mechanical chest inefficiency in people with scoliosis, it was concluded that vital capacity, forced expiratory volume in one second, gas transfer factor, and the maximum static expiratory airway pressure was significantly reduced. The forced inspiratory pressure and total lung capacity were lower in abnormal individuals [8]. Respiratory parameters can be measured using motion capture systems by placing photo-reflect markers on the chest wall [9].
The OEP method was introduced as a valid method for chest kinematic analysis which is divided into different parts in different positions [10]. The validity of OEP has been evaluated in healthy subjects in sitting and standing conditions with the protocol of 89 markers in different laboratory conditions (for example, during quiet breathing and exercise). The maximum difference between spirometry and OEP is <4% in all the studies reported. Inter-rater and intra-rater reliability for the OEP method at rest and during moderate-intensity exercise on the ergometer was checked, and it was seen that the intra-class correlation coefficient was >0.75 and the coefficient of variation was less than 10% [11].
Aliverti et al. measured the changes in respiratory volumes using the OEP method in supine and prone positions in normal subjects. They reported that in the supine and prone positions, most of the chest volume changes were distributed in the abdominal part [10].
Chest kinematic evaluation during breathing and evaluations of thoracoabdominal volumes in clinical work and scientific studies allow us to study different diseases from multiple perspectives and to use more appropriate and direct treatment in rehabilitation [7]. This method was used in different positions in healthy people [10]. Accordingly, this study estimates respiratory volumes in scoliosis patients sitting.
Case Presentation
Study population
Two subjects were recruited, including a healthy person and a person with scoliosis. The inclusion criteria were age between 10-20 years, body mass index (BMI) between 18-30 kg/m2 and a Cobb angle >20 degrees for scoliosis. Meanwhile, the exclusion criteria included a history of spinal and chest injuries, and respiratory disease, any surgery for orthopedic disease or scoliosis, a history of pulmonary or neuromuscular disease, or the inability to perform the test correctly. The scoliosis subject was selected from patients referred to Namazi Hospital in Shiraz City, Iran.
The healthy person was selected, matching the scoliosis person based on BMI, height, weight and age.
The subjects were explained the evaluation procedure before the test and signed an informed consent form.
The subjects were requested to sit comfortably and motionlessly for 30 s after providing basic demographic information, calibrating the assessment site, attaching markers to the subject’s trunk and positioning the subject’s hands comfortably on both sides of the subject’s trunk and the thighs. This process was implemented for quiet breathing as well as deep breathing.
Experimental protocol instrumentation
A total of 89 passive spherical photo-reflective markers with a diameter of 9 mm were placed on the trunk by double-sided adhesive (42 markers on the anterior surface, 37 markers on the posterior surface and 10 markers on the lateral surfaces of the trunk). The insertion pattern of markers was based on chest analysis studies conducted in the past [9].
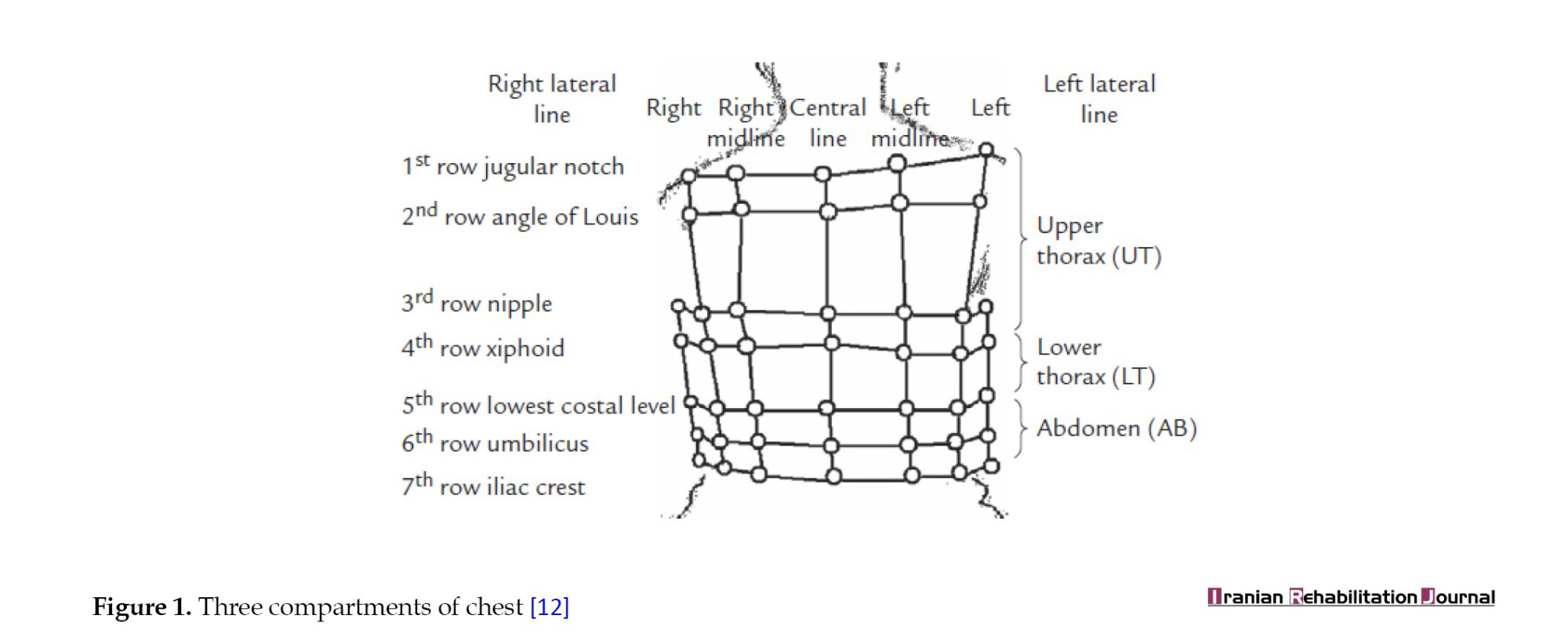
Each marker was followed by motion analysis cameras, including eight high-speed cameras with a frequency of 120 Hz in 3D space. As shown in Figure 1, the chest is divided into three compartments: The upper thoracic compartment, the lower thoracic compartment and the abdominal compartment. It is assumed that the sum of compartment volumes equals the total volume of the chest.
Data analysis
The overall and compartmental volumes were achieved by importing the 3D movement of markers collected by QTM and then importing them into the MOKKA software. Then, the data was entered into MATLAB software, and the final results were obtained. The geometric model made by MATLAB code was created by connecting eight numbers of passive markers and forming a polyhedron and was used to measure pulmonary volumes. The total of the polyhedron volumes inside each compartment served as the basis for calculating the volume of each compartment. Each respiratory cycle’s maximum and lowest volumes were determined, and the discrepancies between them show volume changes during intake and exhalation.
Results
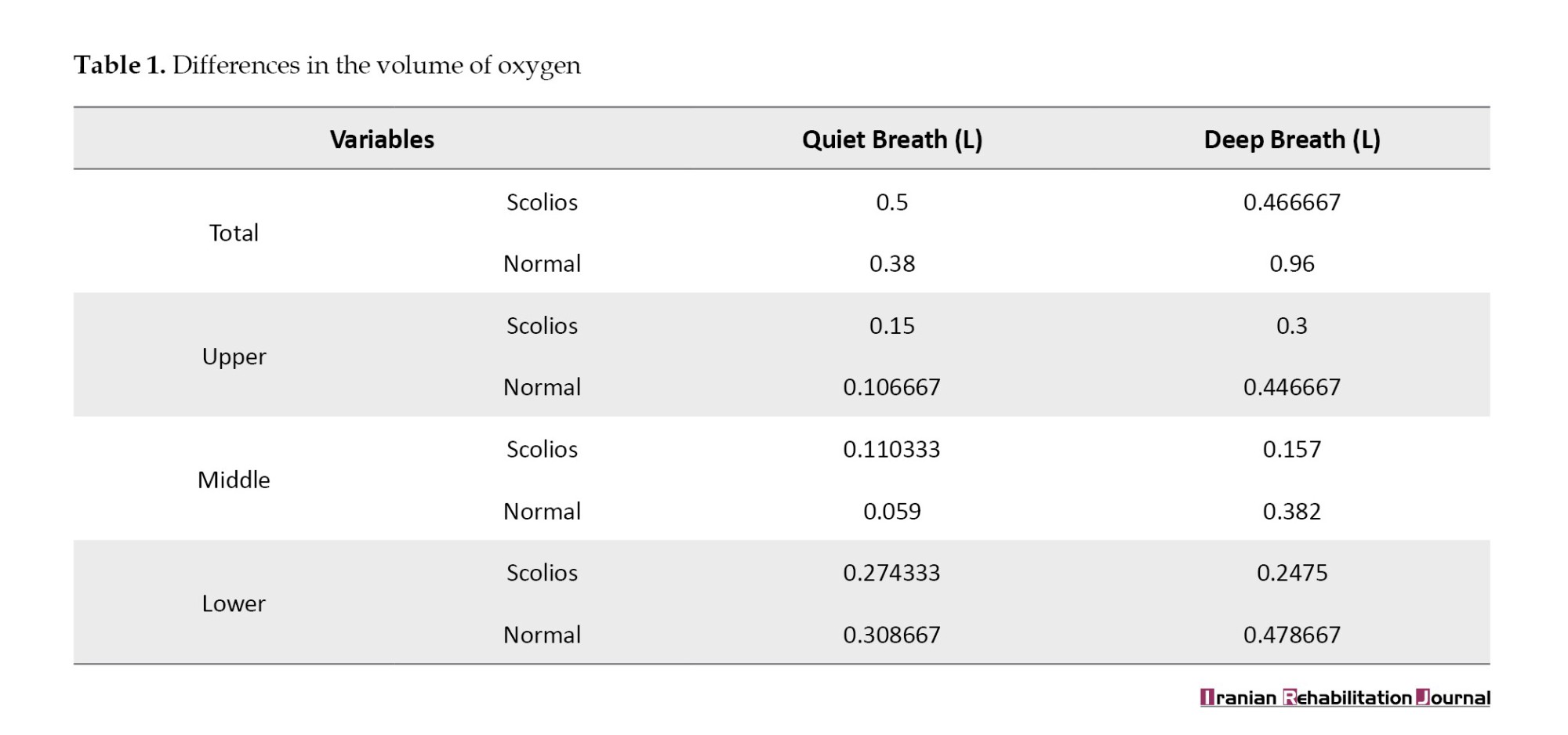
The healthy person was 15 years old, with a height of 165 cm and a weight of 68 kg (BMI=25 kg/m2) and the person with scoliosis was 17 years old, with a height of 167 cm and a weight of 63 kg (BMI=22.6 kg/m2) and with a Cobb angle of 60 degrees. As shown in Table 1, it can be seen in the sitting position in the quiet breathing in a person with scoliosis except in the lower part of the trunk (0.274333 vs 0.308667), total changes in the respiratory volume and changes in respiratory volume in other parts of the trunk is slightly more than those of healthy person (0.5 vs 0.38, 0.15 vs 0.106667, 0.110333 vs 0.059, 0.06667, 0.110333 vs 0.059). However, in deep breathing, the change in respiratory volume in each part of the trunk and the overall change in the respiratory volume in a person with scoliosis is less than healthy (0.466667 vs 0.96, 0.3 vs 0.446667, 0.157 vs 0.382, 0.2475 vs 0.478667).
Discussion
This study investigated respiratory output by OEP in a sitting position in a person with scoliosis and compared it with that of a healthy person. Comparing the results of the study of a healthy person data with scoliosis shows that the changes in the respiratory volume of a scoliosis person in respiration except in the lower thoracic compartment in other compartments are more significant and are more than those of the healthy person. Moreover, the changes in respiratory volume in deep breathing in a healthy person are more in all compartments and overall than those of the patient with scoliosis.
Based on the results, the rate of respiratory volume changes in the upper and middle parts of the chest is higher in the person with scoliosis than those the normal. The person’s reduced reliance on diaphragmatic breathing considered the typical condition of breathing, may be linked to the cause of these additional modifications. Considering spinal and non-symmetrical abnormalities of ribs in persons with scoliosis, as well as lack of proper functioning of respiratory muscles in people with scoliosis, which was proven in studies [13]. People with scoliosis use compensatory mechanisms in respiration, such as rib breathing and this is probably why changes in the volume of the upper parts of the ribs are more common in people with scoliosis than in healthy people.
In people with scoliosis, in terms of trunk deformation, the space between the ribs on the convex and concave sides of the spine is subsequently changed and some intercostal respiratory muscles are strained and others are shortened. This change in muscle length and non-symmetry can affect their function and cause changes in the volume of the upper chest parts of people with scoliosis compared to healthy people [3].
By comparing the changes in the respiratory volume in different parts of the trunk in a healthy person and a person with scoliosis, despite the greater dependence of a person with scoliosis on rib breathing than the healthy person, the rate of changes in the chest volume in the lower part related to diaphragm breathing in both individuals is more than the changes in the upper and middle parts of the chest. These findings may show that diaphragmatic breathing is superior to rib breathing despite the altered respiratory patterns of a person with scoliosis and their increased reliance on rib breathing than healthy persons. In other studies, it was observed that despite the differences in the chest volume in people with scoliosis compared to healthy people, diaphragm movements in both groups are not significantly different [14].
Despite higher maximum respiratory volume in the healthy person in quiet breathing, the changes in respiratory volume are more significant in the person with scoliosis. One of the possible reasons to justify this difference is the presence of motion restriction in the chest of the scoliosis subject; consequently, chest expansion is lower, and finally, with less respiratory volume and the difference in rhythm and respiratory pattern, changes in the respiratory volume of people with scoliosis are more than those of healthy people. Furthermore, due to the lower total lung capacity in people with scoliosis [8], they are likely to use deeper breathing to access sufficient amounts of oxygen and therefore changes in their respiratory volume are more than those of healthy people [15].
The analysis of chest movement is essential for the biomechanical assessments and interaction between muscles and structural components of the respiratory system or for examining respiratory strategies.
Besides, since many diseases, such as neuromuscular and musculoskeletal disorders (muscular dystrophy, spinal diseases, and scoliosis), cause chest movement abnormalities, such analysis can be a valuable tool for diagnosing and measuring the complications of these diseases.
In the past, various tools, such as linear differential transducers, mercury-in rubber strain gauges, magnetometers, and respiratory inductive plethysmography were used to check the movement of the rib, chest, and abdomen [16]. Although in studies, reports on the validity and easy efficiency of chest and abdominal movement assessment were expressed by RIP and MSG systems, 3D changes in the chest cannot be measured using these methods. Moreover, their application is limited to the correct calibration. Several researchers have reported that using these systems without proper calibration was associated with errors. Additionally, in the clinical system, in terms of poor patient cooperation, calibration is complex, and these methods of measuring the 3D volume change of the chest are not suitable [16].
OEP was introduced in studies as a method used in different protocols to evaluate chest changes and measure and analyze respiratory volume in healthy people in different positions [17]. The optoelectronic system for the motion analysis of a large number of reflective markers attached to the body was described by Ferrigno and Pedotti a few years ago [17].
This system can detect small chest movements during breathing with high accuracy. Experimental protocols and computational methods of data obtained from optoelectronic systems were successfully developed to obtain changes in chest volume and its different components via optical measurements of the displacement of points on the outer surface of the chest [16, 18]. This method offers an accurate and reliable estimation of absolute volumes and changes in those volumes during breathing, according to several experiments carried out under various circumstances.
The technique (optoelectronics) measures changes in chest volumes without masks or nasal clips or any restrictive connection to the patient or restrictions on chest freedom of movement during breathing and can evaluate and estimate respiratory volume changes by modeling the surface of thoracoabdominal with a large number of selected anatomical landmarks. One of the advantageous features of the optoelectronic method is the division of chest volume into different compartments. If the left and right parts of the chest are asymmetrical or the function of the respiratory muscles is not symmetrical, the optoelectronic method can measure the expansion of the left and right parts of the chest [16].
The new marking method, the lack of airway constraints compared to conventional techniques of assessing respiratory volumes, and the ability to examine the subject in various contexts while engaging in various physical activities are the benefits of this research. Considering the advantages of the optoelectronic method compared to other respiratory system evaluation methods and the differences seen in these two cases, it is suggested to use this method in a larger society to prove meaningfulness and achieve more accurate results.
Conclusion
The results of comparing the respiratory volumes of a person with scoliosis with a healthy person through the optoelectronic method show that the rate of changes in the respiratory volumes in the middle and lower parts of the chest with scoliosis is higher than in the healthy person. Moreover, the rate of changes in respiratory volumes in all parts of the chest in deep breathing in a healthy person is higher than in a person with scoliosis. OEP can be used without the limitation of movement and without attachment of the mouthpiece and clamp or any tool that informs the person of the purposes of assessment, the volume of the chest in total or by different compartments, and the respiratory output in people with scoliosis.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Bu-Ali Sina University (Code: IR.BASU.REC.14000030).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The researchers thank all participants for their contribution.
References
Scoliosis is a three-dimensional spine deformity characterized by lateral deviation from the normal vertical direction [1, 2]. Scoliosis may occur at any age, without a known cause (idiopathic) or with a known cause [2]. Thoracic spine involvement alone or with the lumbar spine is the primary cause of respiratory or cardiovascular problems [3]. Based on the studies, scoliosis affects respiratory function, including respiratory volumes, chest movements, respiratory muscle strength, respiratory patterns, and respiratory system function during exercise and sleep [2, 4-6].
Scoliosis can cause respiratory disturbances and asymmetry in the chest wall on concave and convex sides. Trunk deformity limits chest mobility, which reduces breathing volume [4].
Regarding the complexity of the relationship among the spine, sternum, and ribs, the displacement and rotation of vertebrae in scoliosis affect the shape of the chest and cause convexity and concavity. Lung inflation will be asymmetrical because the transverse and anteroposterior dimensions of either side of the thorax are substantially different. Furthermore, since the ribs are moving, the chest cavity cannot expand as much as it would otherwise [7].
Various methods can be applied to assess breathing. In particular, spirometry and measuring ventilation gases using a spirometer for measuring respiratory volume are commonly employed. Considering the limitations of these methods, such as using masks and nasal clips and the limitations of use in various conditions, the optoelectronic plethysmography (OEP) method was described as a simple and non-invasive method to measure chest motions and to estimate respiratory volumes [7]. In a study performed by Kotani on chest analysis and diaphragm movements via breathing dynamic magnetic resonance imaging in idiopathic scoliosis patients, it was concluded that chest movements were significantly limited. However, diaphragm movement was normal, and there were correlations between respiratory movements and pulmonary functional tests [5].
In a study conducted by Jones that used the helium dilution closed-circuit technique and the belt restriction method on the mechanical chest inefficiency in people with scoliosis, it was concluded that vital capacity, forced expiratory volume in one second, gas transfer factor, and the maximum static expiratory airway pressure was significantly reduced. The forced inspiratory pressure and total lung capacity were lower in abnormal individuals [8]. Respiratory parameters can be measured using motion capture systems by placing photo-reflect markers on the chest wall [9].
The OEP method was introduced as a valid method for chest kinematic analysis which is divided into different parts in different positions [10]. The validity of OEP has been evaluated in healthy subjects in sitting and standing conditions with the protocol of 89 markers in different laboratory conditions (for example, during quiet breathing and exercise). The maximum difference between spirometry and OEP is <4% in all the studies reported. Inter-rater and intra-rater reliability for the OEP method at rest and during moderate-intensity exercise on the ergometer was checked, and it was seen that the intra-class correlation coefficient was >0.75 and the coefficient of variation was less than 10% [11].
Aliverti et al. measured the changes in respiratory volumes using the OEP method in supine and prone positions in normal subjects. They reported that in the supine and prone positions, most of the chest volume changes were distributed in the abdominal part [10].
Chest kinematic evaluation during breathing and evaluations of thoracoabdominal volumes in clinical work and scientific studies allow us to study different diseases from multiple perspectives and to use more appropriate and direct treatment in rehabilitation [7]. This method was used in different positions in healthy people [10]. Accordingly, this study estimates respiratory volumes in scoliosis patients sitting.
Case Presentation
Study population
Two subjects were recruited, including a healthy person and a person with scoliosis. The inclusion criteria were age between 10-20 years, body mass index (BMI) between 18-30 kg/m2 and a Cobb angle >20 degrees for scoliosis. Meanwhile, the exclusion criteria included a history of spinal and chest injuries, and respiratory disease, any surgery for orthopedic disease or scoliosis, a history of pulmonary or neuromuscular disease, or the inability to perform the test correctly. The scoliosis subject was selected from patients referred to Namazi Hospital in Shiraz City, Iran.
The healthy person was selected, matching the scoliosis person based on BMI, height, weight and age.
The subjects were explained the evaluation procedure before the test and signed an informed consent form.
The subjects were requested to sit comfortably and motionlessly for 30 s after providing basic demographic information, calibrating the assessment site, attaching markers to the subject’s trunk and positioning the subject’s hands comfortably on both sides of the subject’s trunk and the thighs. This process was implemented for quiet breathing as well as deep breathing.
Experimental protocol instrumentation
A total of 89 passive spherical photo-reflective markers with a diameter of 9 mm were placed on the trunk by double-sided adhesive (42 markers on the anterior surface, 37 markers on the posterior surface and 10 markers on the lateral surfaces of the trunk). The insertion pattern of markers was based on chest analysis studies conducted in the past [9].
Each marker was followed by motion analysis cameras, including eight high-speed cameras with a frequency of 120 Hz in 3D space. As shown in Figure 1, the chest is divided into three compartments: The upper thoracic compartment, the lower thoracic compartment and the abdominal compartment. It is assumed that the sum of compartment volumes equals the total volume of the chest.

Data analysis
The overall and compartmental volumes were achieved by importing the 3D movement of markers collected by QTM and then importing them into the MOKKA software. Then, the data was entered into MATLAB software, and the final results were obtained. The geometric model made by MATLAB code was created by connecting eight numbers of passive markers and forming a polyhedron and was used to measure pulmonary volumes. The total of the polyhedron volumes inside each compartment served as the basis for calculating the volume of each compartment. Each respiratory cycle’s maximum and lowest volumes were determined, and the discrepancies between them show volume changes during intake and exhalation.
Results
The healthy person was 15 years old, with a height of 165 cm and a weight of 68 kg (BMI=25 kg/m2) and the person with scoliosis was 17 years old, with a height of 167 cm and a weight of 63 kg (BMI=22.6 kg/m2) and with a Cobb angle of 60 degrees. As shown in Table 1, it can be seen in the sitting position in the quiet breathing in a person with scoliosis except in the lower part of the trunk (0.274333 vs 0.308667), total changes in the respiratory volume and changes in respiratory volume in other parts of the trunk is slightly more than those of healthy person (0.5 vs 0.38, 0.15 vs 0.106667, 0.110333 vs 0.059, 0.06667, 0.110333 vs 0.059). However, in deep breathing, the change in respiratory volume in each part of the trunk and the overall change in the respiratory volume in a person with scoliosis is less than healthy (0.466667 vs 0.96, 0.3 vs 0.446667, 0.157 vs 0.382, 0.2475 vs 0.478667).

Discussion
This study investigated respiratory output by OEP in a sitting position in a person with scoliosis and compared it with that of a healthy person. Comparing the results of the study of a healthy person data with scoliosis shows that the changes in the respiratory volume of a scoliosis person in respiration except in the lower thoracic compartment in other compartments are more significant and are more than those of the healthy person. Moreover, the changes in respiratory volume in deep breathing in a healthy person are more in all compartments and overall than those of the patient with scoliosis.
Based on the results, the rate of respiratory volume changes in the upper and middle parts of the chest is higher in the person with scoliosis than those the normal. The person’s reduced reliance on diaphragmatic breathing considered the typical condition of breathing, may be linked to the cause of these additional modifications. Considering spinal and non-symmetrical abnormalities of ribs in persons with scoliosis, as well as lack of proper functioning of respiratory muscles in people with scoliosis, which was proven in studies [13]. People with scoliosis use compensatory mechanisms in respiration, such as rib breathing and this is probably why changes in the volume of the upper parts of the ribs are more common in people with scoliosis than in healthy people.
In people with scoliosis, in terms of trunk deformation, the space between the ribs on the convex and concave sides of the spine is subsequently changed and some intercostal respiratory muscles are strained and others are shortened. This change in muscle length and non-symmetry can affect their function and cause changes in the volume of the upper chest parts of people with scoliosis compared to healthy people [3].
By comparing the changes in the respiratory volume in different parts of the trunk in a healthy person and a person with scoliosis, despite the greater dependence of a person with scoliosis on rib breathing than the healthy person, the rate of changes in the chest volume in the lower part related to diaphragm breathing in both individuals is more than the changes in the upper and middle parts of the chest. These findings may show that diaphragmatic breathing is superior to rib breathing despite the altered respiratory patterns of a person with scoliosis and their increased reliance on rib breathing than healthy persons. In other studies, it was observed that despite the differences in the chest volume in people with scoliosis compared to healthy people, diaphragm movements in both groups are not significantly different [14].
Despite higher maximum respiratory volume in the healthy person in quiet breathing, the changes in respiratory volume are more significant in the person with scoliosis. One of the possible reasons to justify this difference is the presence of motion restriction in the chest of the scoliosis subject; consequently, chest expansion is lower, and finally, with less respiratory volume and the difference in rhythm and respiratory pattern, changes in the respiratory volume of people with scoliosis are more than those of healthy people. Furthermore, due to the lower total lung capacity in people with scoliosis [8], they are likely to use deeper breathing to access sufficient amounts of oxygen and therefore changes in their respiratory volume are more than those of healthy people [15].
The analysis of chest movement is essential for the biomechanical assessments and interaction between muscles and structural components of the respiratory system or for examining respiratory strategies.
Besides, since many diseases, such as neuromuscular and musculoskeletal disorders (muscular dystrophy, spinal diseases, and scoliosis), cause chest movement abnormalities, such analysis can be a valuable tool for diagnosing and measuring the complications of these diseases.
In the past, various tools, such as linear differential transducers, mercury-in rubber strain gauges, magnetometers, and respiratory inductive plethysmography were used to check the movement of the rib, chest, and abdomen [16]. Although in studies, reports on the validity and easy efficiency of chest and abdominal movement assessment were expressed by RIP and MSG systems, 3D changes in the chest cannot be measured using these methods. Moreover, their application is limited to the correct calibration. Several researchers have reported that using these systems without proper calibration was associated with errors. Additionally, in the clinical system, in terms of poor patient cooperation, calibration is complex, and these methods of measuring the 3D volume change of the chest are not suitable [16].
OEP was introduced in studies as a method used in different protocols to evaluate chest changes and measure and analyze respiratory volume in healthy people in different positions [17]. The optoelectronic system for the motion analysis of a large number of reflective markers attached to the body was described by Ferrigno and Pedotti a few years ago [17].
This system can detect small chest movements during breathing with high accuracy. Experimental protocols and computational methods of data obtained from optoelectronic systems were successfully developed to obtain changes in chest volume and its different components via optical measurements of the displacement of points on the outer surface of the chest [16, 18]. This method offers an accurate and reliable estimation of absolute volumes and changes in those volumes during breathing, according to several experiments carried out under various circumstances.
The technique (optoelectronics) measures changes in chest volumes without masks or nasal clips or any restrictive connection to the patient or restrictions on chest freedom of movement during breathing and can evaluate and estimate respiratory volume changes by modeling the surface of thoracoabdominal with a large number of selected anatomical landmarks. One of the advantageous features of the optoelectronic method is the division of chest volume into different compartments. If the left and right parts of the chest are asymmetrical or the function of the respiratory muscles is not symmetrical, the optoelectronic method can measure the expansion of the left and right parts of the chest [16].
The new marking method, the lack of airway constraints compared to conventional techniques of assessing respiratory volumes, and the ability to examine the subject in various contexts while engaging in various physical activities are the benefits of this research. Considering the advantages of the optoelectronic method compared to other respiratory system evaluation methods and the differences seen in these two cases, it is suggested to use this method in a larger society to prove meaningfulness and achieve more accurate results.
Conclusion
The results of comparing the respiratory volumes of a person with scoliosis with a healthy person through the optoelectronic method show that the rate of changes in the respiratory volumes in the middle and lower parts of the chest with scoliosis is higher than in the healthy person. Moreover, the rate of changes in respiratory volumes in all parts of the chest in deep breathing in a healthy person is higher than in a person with scoliosis. OEP can be used without the limitation of movement and without attachment of the mouthpiece and clamp or any tool that informs the person of the purposes of assessment, the volume of the chest in total or by different compartments, and the respiratory output in people with scoliosis.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Bu-Ali Sina University (Code: IR.BASU.REC.14000030).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The researchers thank all participants for their contribution.
References
- Yalfani A, Bazipoor P. The effects of adolescent idiopathic scoliosis on the factors affecting the respiratory system and its function (A Systematic Review). Journal of Rehabilitation Sciences & Research. 2020; 7(1):1-7. [Link]
- Kim W, Porrino JA, Hood KA, Chadaz TS, Klauser AS, Taljanovic MS. Clinical evaluation, imaging, and management of adolescent idiopathic and adult degenerative scoliosis. Current Problems in Diagnostic Radiology. 2019; 48(4):402-14. [DOI:10.1067/j.cpradiol.2018.08.006] [PMID]
- Koumbourlis AC. Scoliosis and the respiratory system. Paediatric Respiratory Reviews. 2006; 7(2):152-60. [DOI:10.1016/j.prrv.2006.04.009] [PMID]
- Kim MJ, Park DS. The effect of Schroth’s three-dimensional exercises in combination with respiratory muscle exercise on Cobb’s angle and pulmonary function in patients with idiopathic scoliosis. Physical Therapy Rehabilitation Science. 2017; 6(3):113-9. [DOI:10.14474/ptrs.2017.6.3.113]
- Kotani T, Minami S, Takahashi K, Isobe K, Nakata Y, Takaso M, et al. An analysis of chest wall and diaphragm motions in patients with idiopathic scoliosis using dynamic breathing MRI. Spine. 2004; 29(3):298-302. [DOI:10.1097/01.BRS.0000106490.82936.89] [PMID]
- Li X, Guo H, Chen C, Tan H, Lin Y, Li Z, et al. Does scoliosis affect sleep breathing? World Neurosurgery. 2018; 118:e946-e50. [DOI:10.1016/j.wneu.2018.07.106] [PMID]
- Massaroni C, Carraro E, Vianello A, Miccinilli S, Morrone M, Levai IK, et al. Optoelectronic plethysmography in clinical practice and research: A review. Respiration. 2017; 93(5):339-54. [DOI:10.1159/000462916] [PMID]
- Jones R, Kennedy J, Hasham F, Owen R, Taylor J. Mechanical inefficiency of the thoracic cage in scoliosis. Thorax. 1981; 36(6):456-61. [DOI:10.1136/thx.36.6.456] [PMID]
- Massaroni C, Senesi G, Schena E, Silvestri S. Analysis of breathing via optoelectronic systems: comparison of four methods for computing breathing volumes and thoraco-abdominal motion pattern. Computer Methods in Biomechanics and Biomedical Engineering. 2017; 20(16):1678-89. [DOI:10.1080/10255842.2017.1406081] [PMID]
- Aliverti A, Dellacà R, Pelosi P, Chiumello D, Gattinoni L, Pedotti A. Compartmental analysis of breathing in the supine and prone positions by optoelectronic plethysmography. Annals of Biomedical Engineering. 2001; 29(1):60-70. [DOI:10.1114/1.1332084] [PMID]
- Vieira DS, Hoffman M, Pereira DA, Britto RR, Parreira VF. Optoelectronic plethysmography: Intra-rater and inter-rater reliability in healthy subjects. Respiratory Physiology & Neurobiology. 2013; 189(3):473-6. [DOI:10.1016/j.resp.2013.08.023] [PMID]
- Wang HK, Lu TW, Liing RJ, Shih TT, Chen SC, Lin KH. Relationship between chest wall motion and diaphragmatic excursion in healthy adults in the supine position. Journal of the Formosan Medical Association. 2009; 108(7):577-86. [DOI:10.1016/S0929-6646(09)60376-4] [PMID]
- Mohammadi P, Akbari M, Sarafzadeh J. Comparison of respiratory muscle electromyography between adolescent idiopathic scoliosis and healthy subjects. Physical Treatments-Specific Physical Therapy Journal. 2014; 4(1):33-8. [Link]
- Sperandio EF, Vidotto MC, Alexandre AS, Yi LC, Gotfryd AO, Dourado VZ. Functional exercise capacity, lung function and chest wall deformity in patients with adolescent idiopathic scoliosis. Fisioterapia em Movimento. 2015; 28:563-72.[DOI: 10.1590/0103-5150.028.003.AO15]
- Flores F, Cavaleiro J, Lopes A, Ribeiro F, Oliveira A. Preoperative pulmonary function and respiratory muscle strength in Portuguese adolescents with idiopathic scoliosis. Revista Portuguesa de Pneumologia. 2016; 22(1):52-3. [DOI:10.1016/j.rppnen.2015.09.003] [PMID]
- Aliverti A, Pedotti A. Opto-electronic plethysmography. In: Aliverti A, Brusasco V, Macklem PT, Pedotti A, editors. Mechanics of breathing. Milano: Springer; 2002. [DOI:10.1007/978-88-470-2916-3_5]
- Ferrigno G, Pedotti A. ELITE: A digital dedicated hardware system for movement analysis via real-time TV signal processing. IEEE Transactions on Biomedical Engineering. 1985; 32(11):943-50. [DOI:10.1109/TBME.1985.325627] [PMID]
- Ferrigno G, Carnevali P, Aliverti A, Molteni F, Beulcke G, Pedotti A. Three-dimensional optical analysis of chest wall motion. Journal of Applied Physiology. 1994; 77(3):1224-31. [DOI:10.1152/jappl.1994.77.3.1224] [PMID]
Article type: Case Reports |
Subject:
methodology in rehabilitation
Received: 2023/02/28 | Accepted: 2023/07/25 | Published: 2024/06/1
Received: 2023/02/28 | Accepted: 2023/07/25 | Published: 2024/06/1
Send email to the article author








