Volume 21, Issue 4 (December 2023)
Iranian Rehabilitation Journal 2023, 21(4): 663-694 |
Back to browse issues page
Ethics code: ADM/DCST/HREC/APP/2638
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nicholas Oghumu S, Okafor U A C, Salu O B, Tella B A. Effects of Lumbar Stabilization and Graded Activity Exercises on Selected Biochemical Mediators and Clinical Outcomes in Patients With Non-specific Chronic Low Back Pain. Iranian Rehabilitation Journal 2023; 21 (4) :663-694
URL: http://irj.uswr.ac.ir/article-1-1930-en.html
URL: http://irj.uswr.ac.ir/article-1-1930-en.html
Saturday Nicholas Oghumu * 

 1, Udoka Arinze Chris Okafor2
1, Udoka Arinze Chris Okafor2 

 , Olumuyiwa Babalola Salu3
, Olumuyiwa Babalola Salu3 

 , Bosede Abidemi Tella4
, Bosede Abidemi Tella4 




 1, Udoka Arinze Chris Okafor2
1, Udoka Arinze Chris Okafor2 

 , Olumuyiwa Babalola Salu3
, Olumuyiwa Babalola Salu3 

 , Bosede Abidemi Tella4
, Bosede Abidemi Tella4 


1- Department of Physiotherapy, School of Postgraduate Studies, University of Lagos, Lagos, Nigeria.
2- Department of Physiotherapy, Faculty of Clinical Sciences, College of Medicine, University of Lagos, Lagos, Nigeria.
3- Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria.
4- Department of Physiotherapy, Faculty of Allied Medical Sciences, College of Medical Sciences, University of Calabar, Calabar, Nigeria.
2- Department of Physiotherapy, Faculty of Clinical Sciences, College of Medicine, University of Lagos, Lagos, Nigeria.
3- Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria.
4- Department of Physiotherapy, Faculty of Allied Medical Sciences, College of Medical Sciences, University of Calabar, Calabar, Nigeria.
Keywords: Stabilization exercise, Graded activity exercise, Biochemical mediators, Clinical outcomes, Chronic low-back pain
Full-Text [PDF 10465 kb]
(311 Downloads)
| Abstract (HTML) (1100 Views)
Full-Text: (164 Views)
Introduction
Non-specific chronic low back pain (NSCLBP) represents the vast majority of chronic low back pain (CLBP) complaints consulted by physiotherapists [1]. It is defined as CLBP without a definite and recognizable primary disease, persisting for at least three months with clinical signs of muscle tension or stiffness localized below the costal margin of the spine and above the inferior gluteal folds in the presence or absence of sciatica [1]. The impact of NSCLBP on individuals and society is enormous because studies showed that it results in significant health burden, disability, absence from work, high cost of management, reduced quality of life, and increased psychological variables of anxiety with dissatisfaction and hopelessness [1-4].
Moreover, several treatment approaches for NSCLBP have evolved with differing principles at the centre of treatment algorithms [1, 2]. Therapeutic exercises are the first line of choice among different treatments for NSCLBP [1, 5]. Exercise therapy is effective for the management of NSCLBP resulting in improved clinical outcomes of pain, disability, and quality of life [1, 5, 6]. Also, among exercise therapies for NSCLBP, lumbar stabilization exercise (LSE) and graded activity exercises (GAE) are reported to be effective in reducing pain and disability [6]. LSE gained significant interest among CLBP researchers following the evidence that it activates the deep trunk muscles and restores synergic actions of the deep and superficial trunk muscles while reducing pain and disability in patients with NSCLBP [7, 8]. On the other hand, graded activity exercise addresses pain associated with fear of movement, unhelpful beliefs, and behavioral adaptations of NSCLBP while restoring dysfunctional muscle strength, endurance, and balance [9]. Interventionally, LSE emphasizes core stabilization using progressing strength and endurance exercises, while GAE uses behavioral quotas, pacing, and positive reinforcement as psychological constructs to enhance the muscle strength, endurance, and posture of patients with NSCLBP [9, 10, 11].
However, further studies on LSE and GAE in patients with NSCLBP are necessary because reports implicate numerous biochemical mediators in patients with NSCLBP [12-14]. Emerging evidence of pain mechanisms in NSCLBP suggests the need to profile biochemical mediators of pain for optimum intervention [3, 12]. Biochemically, the pain response is associated with the presence of inflammatory mediators of bradykinin, serotonin, histamine, adenosine triphosphate, prostaglandins, nitric oxides, cytokines, leukotrienes, cyclooxygenases, and neurotrophins [15]. Elevated levels of some biochemical mediators of pain have been reported in patients with NSCLBP [13, 16, 17, 18]. The cellular potentiation of some biochemical mediators is reported to influence the persistence of chronic pain, such as NSCLBP [18]. Also, studies support the evaluation of biochemical mediators of interleukin (IL)-6, tumor necrosis factor-alpha, interleukin 1A (IL1A), interleukin 18 receptor 1 (IL18R1), interleukin 18 receptor accessory protein (IL18RAP), cyclooxygenase 2 (COX2) and matrix metalloprotease 3 given their association with disc degeneration, pain intensity, and disability in patients with NSCLBP [16, 17, 19, 20].
Consequently, evaluating biochemical mediators of pain as part of treatment outcome is desirable since recommendations are presented for favourable treatments in NSCLBP. When implemented as part of treatment outcome, the results from such studies help broaden and refine the clinical significance of recommended treatments. Along with issues of exercise superiority [21], few studies examine the effects of therapeutic exercises on biochemical mediators in patients with CLBP with promising results. The available evidence suggests the state of inducible immune activation after therapeutic exercises in patients with LBP [21, 22, 23, 24]. Al-Obaidi and Mahmoud studied McKenzie’s exercise on immune response in acute LBP [23], while Sokunbi et al. examined the effects of LSE on serotonin in CLBP [22]. Minobes-Molina et al. investigated the effect of multimodal treatments, including exercise therapies on IL-6 concentrations in NSCLBP [24]. Thus, few or no studies examine the effects of only therapeutic exercise on immune response in NSCLBP. Therefore, this study was designed to evaluate the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, IL6 COX2 and clinical outcomes of pain intensity, disability, catastrophizing, diverting attention, cognitive coping with pain reinterpretation in patients with NSCLBP. This study hypothesized that the effects of LSE and GAE on concentrations of selected cytokines and clinical outcomes are not significantly different.
Materials and Methods
Study design
The study was a single-blind parallel trial with an adaptive trial design. The study was registered with the Pan African Clinical Trial Registry. The trial was conducted in two phases, before which a feasibility study was conducted. Phase 1 obtained the baseline, age, and gender values of IL1A, IL18R1, IL18RAP, IL-6, and COX2 in patients with NSCLBP as reported by Oghumu et al. [25]. Also, phase 1 ascertained the responsiveness of IL1A, IL18R1, IL18RAP, IL-6, and COX2 to LSE and GAE for 10 weeks in patients with NSCLBP using an interim analysis. Phase 2 evaluated the effects of LSE and GAE on the responsive biochemical mediator (s) and the selected clinical outcomes in patients with NSCLBP.
Sampling technique and selection criteria
Consecutive sampling was used to recruit all participants. Participants’ selection criteria were hinged on commonly reported domains for inclusion criteria in NSCLBP studies and the mean half-life of commonly used analgesics [26, 27]. The inclusion criteria included patients referred for only NSCLBP with or without radiating symptoms of at least three months, patients having no other site of pain, patients aged 18 to 60 years, and healthy individuals with no history of LBP in the last six months. The exclusion criteria included patients with a diagnosis of spinal inflammatory disease, such as ankylosing spondylitis, a history of spinal fracture or dislocation, motor or sensory deficit, pregnancy, any systemic or medical condition, such as diabetes, hematological disorder, acute or chronic liver diseases, autoimmune disease, use of oral or topical pain medications (non-steroidal anti-inflammatory drugs, steroid or any form of analgesics within the last two days before presentation), intention not to stop the analgesics during the study, a history of psychotropic medications, such as benzodiazepine, and open wound in any part of the body. Patients and healthy participants with a history of smoking and drinking alcohol in the last six months were excluded from the study.
Randomization, study groups, and blinding of participants
Patients with NSCLBP were randomly allocated to LSE and GAE by computer-generated block sizes varying between 4 and 8. Three groups existed in each phase of the trial. Group 1 was patients who received LSE, Group 2 was patients who received GAE, and Group 3 was healthy participants who received no treatment. Treatment appointments were scheduled for patients on different days to ensure blinding.
Determination of sample size
Oghumu et al. reported that in phase 1, 16 patients with NSCLBP and 16 healthy participants were calculated [25]. Each treatment group in phase 1 had 8 patients and 6 patients completed the treatments. For phase 2, Chan’s formula (Equation 1) was used, which assumed a type 1 error and a power of 80% [28].
1. n=c×π1(1-π1)+π2(1-π2)/(π1-π2)2
Given a two-sided test of 5%, the formula assumes that a successful outcome of 25% in one intervention will only be relevant if we observe a 40% effect size of absolute improvement in the other intervention. Therefore, given that n=sample size, π1=0.25, π2=0.65, c depends on the power of the sample (c=7.9 for a power of 80%), n=7.9×0.25(1-0.25)+0.65(1-0.65)/(0.25-0.65)2=21. For three groups, n=3×21=63. Assuming a 20% drop-out, n=63×1.25=78.75. Hence, 54 eligible patients with NSCLBP were randomly assigned to the treatment groups (Figure 1). Also, 27 volunteered healthy participants were recruited as control for baseline values.
Procedure for data collection
The same procedure was employed for the two phases of the trial. Participants’ history and physical assessment were performed by the researcher to ascertain the inclusion and exclusion criteria. Then participants were given commencement dates for the study. On the first day of the participants’ arrival, 8.5 mL of blood was drawn from their right arm by a phlebotomist into test tubes containing ethylenediaminetetraacetic acid as described by Vaught and Henderson [29]. All blood samples were taken at 11 AM. The test tubes were preserved at +4°C in a container filled with cold packs and transported to a central research laboratory where the blood samples were processed and stored at -80°C for analysis.
Participants’ age and gender were obtained, heights were measured in meters (m) with the health-0-meter incorporated, Bridgeview, Illinois, United States of America, weights in kilograms (kg) with the Hana scale (model BR-9011-Germany), and percentage body fats in % with the Omron BF306 monitor. The body mass index (BMI) of participants was estimated in kg/m2. Also, patients’ clinical outcomes were assessed with the following instruments, pain intensity with a visual analogue scale (VAS), disability with a Roland Morris disability questionnaire 24 (RMDQ24), catastrophizing, diverting attention, cognitive coping, and pain reinterpretation with a coping strategy questionnaire 24 (CSQ24), respectively.
Treatments were administered two times a week for 10 weeks of 20 sessions. Post-treatment concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 were assessed on the first day, fifth and tenth weeks, respectively. Also, post-treatment pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation were assessed in the fifth and tenth weeks, respectively.
Treatment procedures
The principles of exercise prescription were followed for LSE and GAE. Stretching exercises were performed before the LSE and GAE. Stretching was only indicated for observed tight muscles. A session of LSE or GAE lasted at least 45 minutes at three sets of repetitions. The abdominal drawing-in maneuver (Figure 2) was incorporated in all phases of the LSE, while cognitive behavioural therapy and bicycle ergometry were used in all phases of GAE (Figure 3). Phase 1 of the trial was conducted for 7 months, while phase 2 was conducted for 11 months.
Stretching exercises
Stretching exercises were performed on tight iliopsoas, rectus femoris, piriformis, and hamstrings of the lower extremities. Stretches were held for 30 s and repeated thrice.
Lumbar stabilization exercise (LSE)
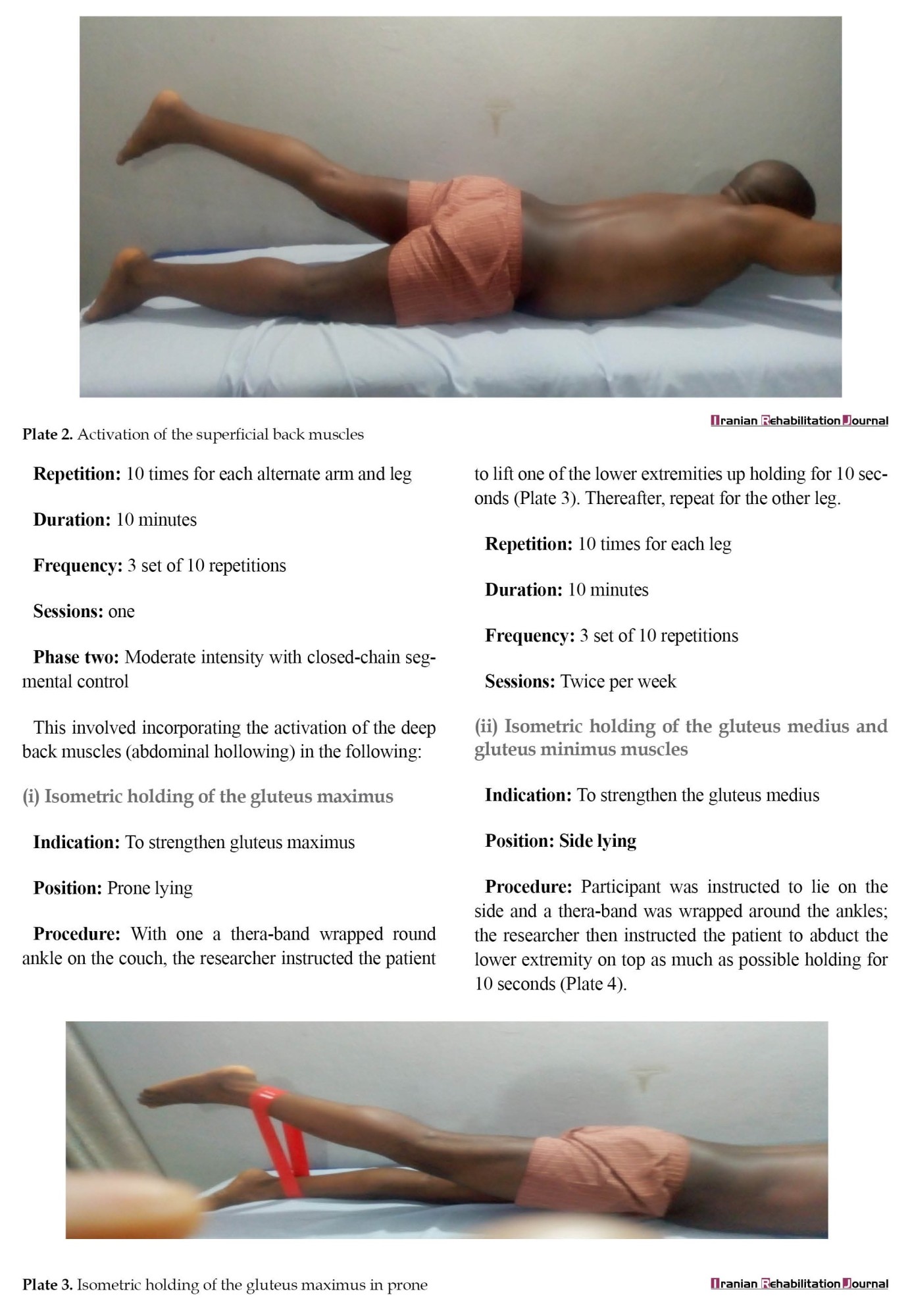
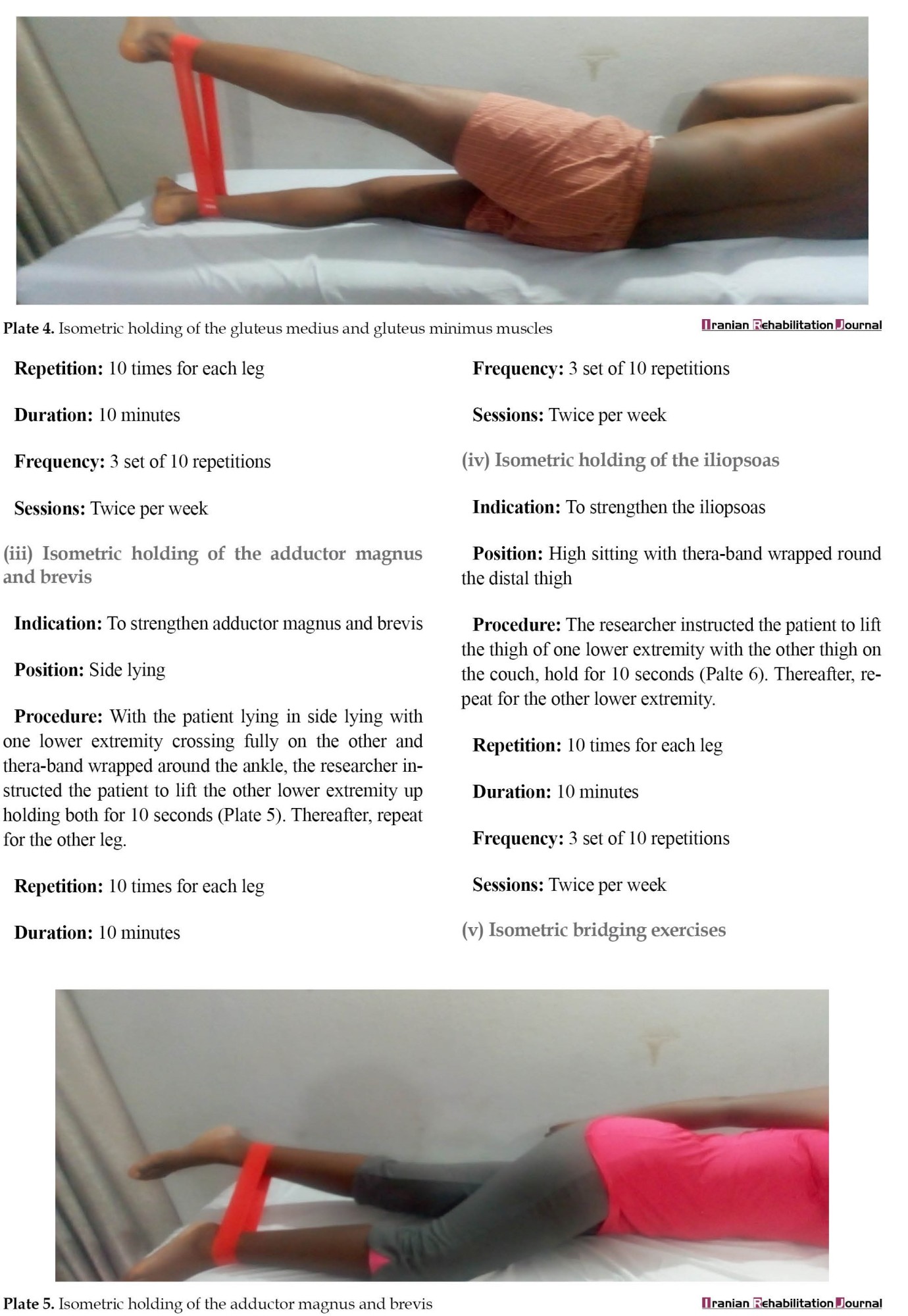
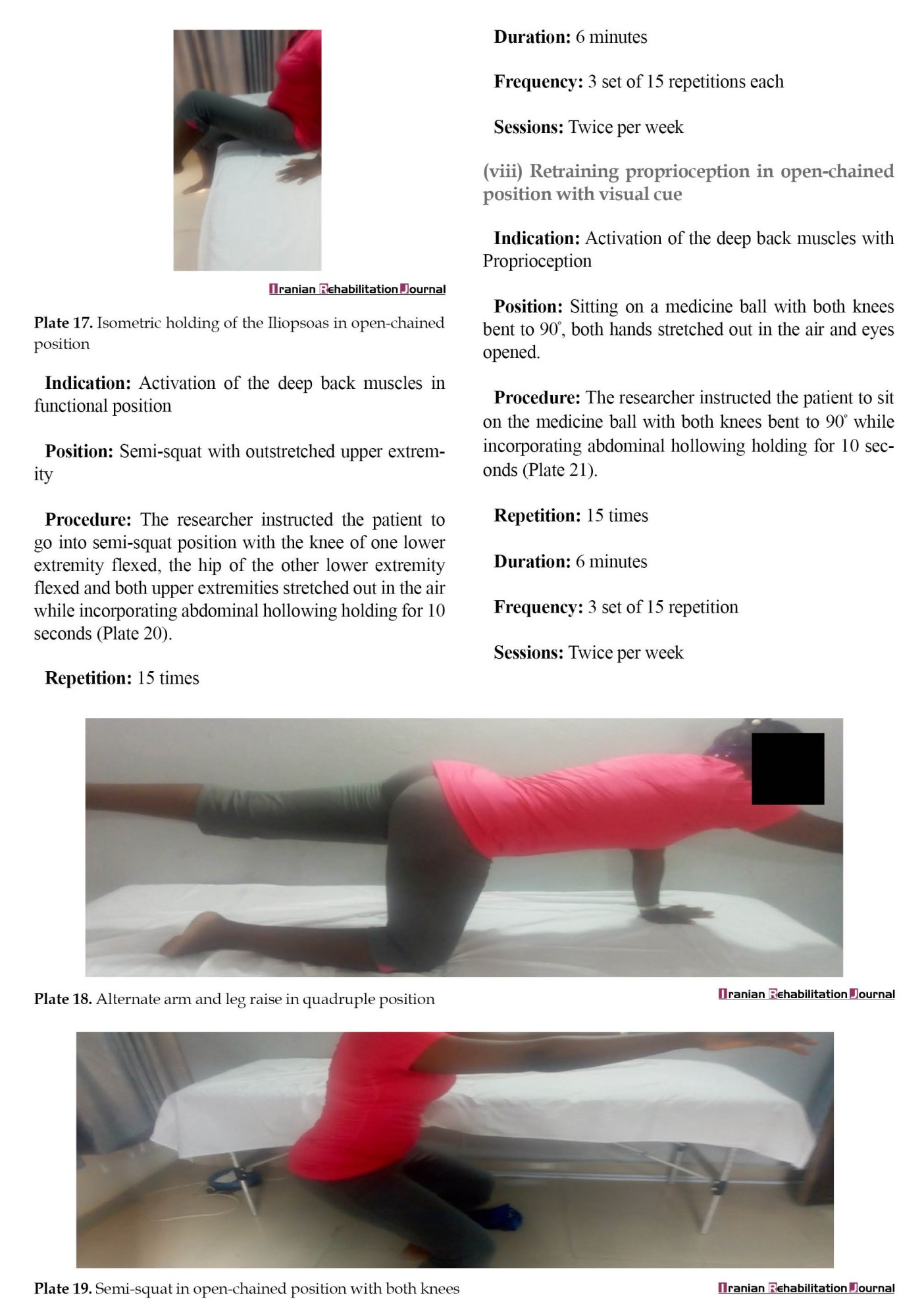
LSE was based on the treatment program described in a previous study [30]. Three phases of the LSE program existed (Appendix). Phase 1 of the LSE was conducted for one session and it involved contraction of the transversus abdominis, multifidus, pelvic floor muscles, and diaphragm. It also involved isometric contraction of the erector spinalis and gluteus maximus in prone lying. Phase 2 LSE was conducted for 5 weeks and consisted of moderate-intensity (10 repetitions) closed-chain isometric strengthening of the gluteus maximus in prone, gluteus medius and minimus in side-lying, adductor magnus and brevis, iliopsoas in high sitting, quadriceps in supine and bridging exercise. It also involved strengthening exercises in functional position on all fours, full and semi-squatting, and proprioception training. Phase 3 of LSE was conducted for 5 weeks and consisted of high-intensity exercises (15 repetitions) in open-chain involving isometric strengthening of the same muscles in phase 2. It also involved isometric strengthening in open-chain functional positions on all fours, full and semi-squatting, and proprioception training.
Graded activity exercise (GAE)
GAE was based on the treatment program described by previous studies [9, 30]. The GAE aimed to increase patients’ activity tolerance using individualized and sub-maximal exercises with cognitive behavioral principles, such as ignoring pain, pacing, explaining pain mechanisms, and reinforcing wellness behavior of exercise benefits. The individualized and sub-maximal exercises included isometric strengthening exercises to the quadriceps femoris, hamstring, gluteus maximus, gluteus medius and minimus, erector spinae, abdominal muscles, and bicycle ergometry (Appendix). The GAE was performed in three phases of sub-maximal exercises. Phases 1 and 2 were moderate-intensity exercises (10 repetitions) with 2 kg weights, while phase 3 consisted of high intensity (15 repetitions) with 3 kg weights. Phase 1 of the GAE lasted for one session, while phases 2 and 3 lasted for 5 weeks each.
Outcome measures
Visual analogue scale (VAS)
The VAS was represented by a 10 cm horizontal line. The horizontal line is anchored with no pain at one end and very extreme pain at the other end [31]. Patients were instructed to indicate their pain intensity by placing a vertical line on the horizontal line. The VAS is reported to have high reliability and validity scores [31].
Roland morris disability questionnaire 24 (RMDQ24)
The RMDQ24 has 24 items on physical functions likely to be affected by low back pain [32]. Its score involves adding up the number of items checked. The scores ranged from 0 (no disability) to 24 (maximum disability) [32]. The RMDQ24 has good construct validity with Cronbach’s scores ranging from 0.7 to 0.90 [32].
Coping strategy questionnaire 24 (CSQ24)
The CSQ24 consists of 23 items about coping strategies [33]. It has a 7-point Likert scale ranging from “never do that” and “always do that,” and one item measuring perceived control over pain with a 7-point Likert scale ranging from “no control” and “complete control [33].” It has four subscales, catastrophizing, diverting attention, reinterpreting, and cognitive coping. Each subscale is scored from zero to 36. The CSQ24 has a good level of validity [33].
Enzyme-linked immunosorbent assay (ELISA)
The ELISA was used to quantify the concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 in the collected blood samples, as described by Chiswick et al. [34]. The ELISA follows the same procedure, as described by Oghumu et al. [25] in phase 1 of the trial. The ELISA is very sensitive and valid to detect and measure proteins on the pictogram scale [35].
Data analysis
Data were analyzed using IBM SPSS software, Version 26. Data for concentrations of IL1A, IL18R1, IL-6, IL18RAP, and COX2 were log-transformed as recommended by the US center for disease control and prevention [36]. Descriptive statistics summarized the Mean±SD and percentages of the data. The Bayesian one-way repeated measure analysis of variance (ANOVA) was used to evaluate the responsiveness of the concentrations of IL1A, IL18R1, IL6, IL18RAP, and COX2 to LSE and GAE in phase 1 of the trial. The previously stated evidential categories of Bayes factor (BF10) reported in the literature were used to inform the decision to respond [37].
Inferential statistics of Kruskal-Wallis one-way ANOVA tested the significant difference among study groups. The Mann-Whitney U test tested the significant difference in IL-6 concentrations between study groups. An independent t-test was used to test the significant difference in baseline values of clinical outcomes between treatment groups. The Friedman test determined the effects of LSE and GAE on patients’ concentrations of IL-6. Also, the Wilcoxon test determined the within-group comparison of treatment effects of LSE and GAE on patients’ concentrations of IL-6. One-way repeated measure ANOVA determined the treatment effects of LSE and GAE on patients’ pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation. The 95% CI was used for mean differences. The significance level was set at P<0.05.
Results
Sixteen patients with NSCLBP (56.25% men and 43.75% women) and 14 healthy individuals (50% men and 50% women) participated in phase 1 of the trial. Table 1 and Table 2 present the response of concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 to LSE and GAE in phase 1 of the trial, respectively.
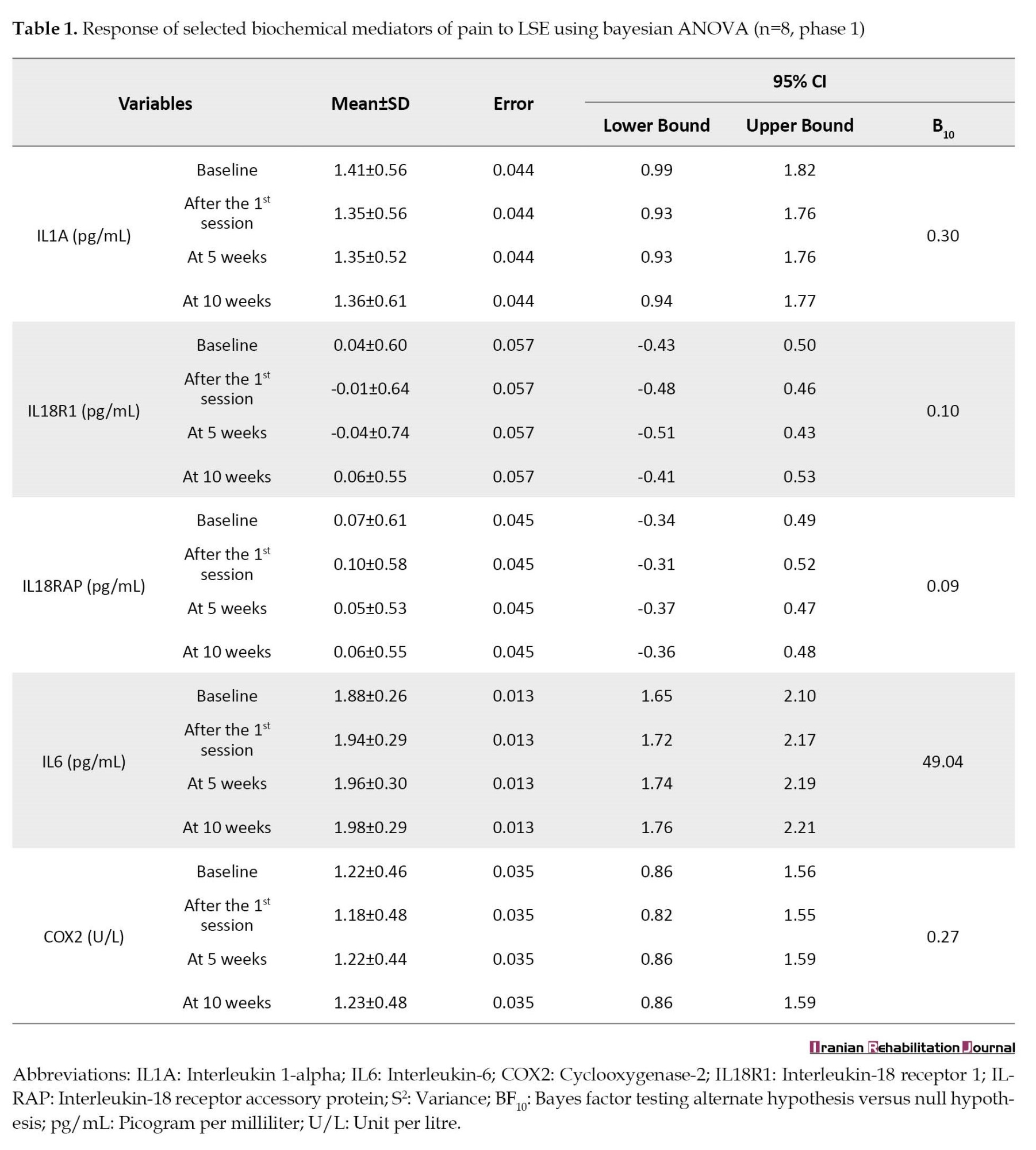

The result revealed that the concentrations of IL1A, IL18R1, IL18RAP, and COX2 were not responsive to LSE and GAE (BF10<1) after 10 weeks of treatments, while the concentrations of IL-6 were responsive (BF10>1) (Table 1 and Table 2). Hence, the trial on the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, and COX2 was stopped, while the trial on the effects of LSE and GAE on concentrations of IL-6 continued in phase 2.
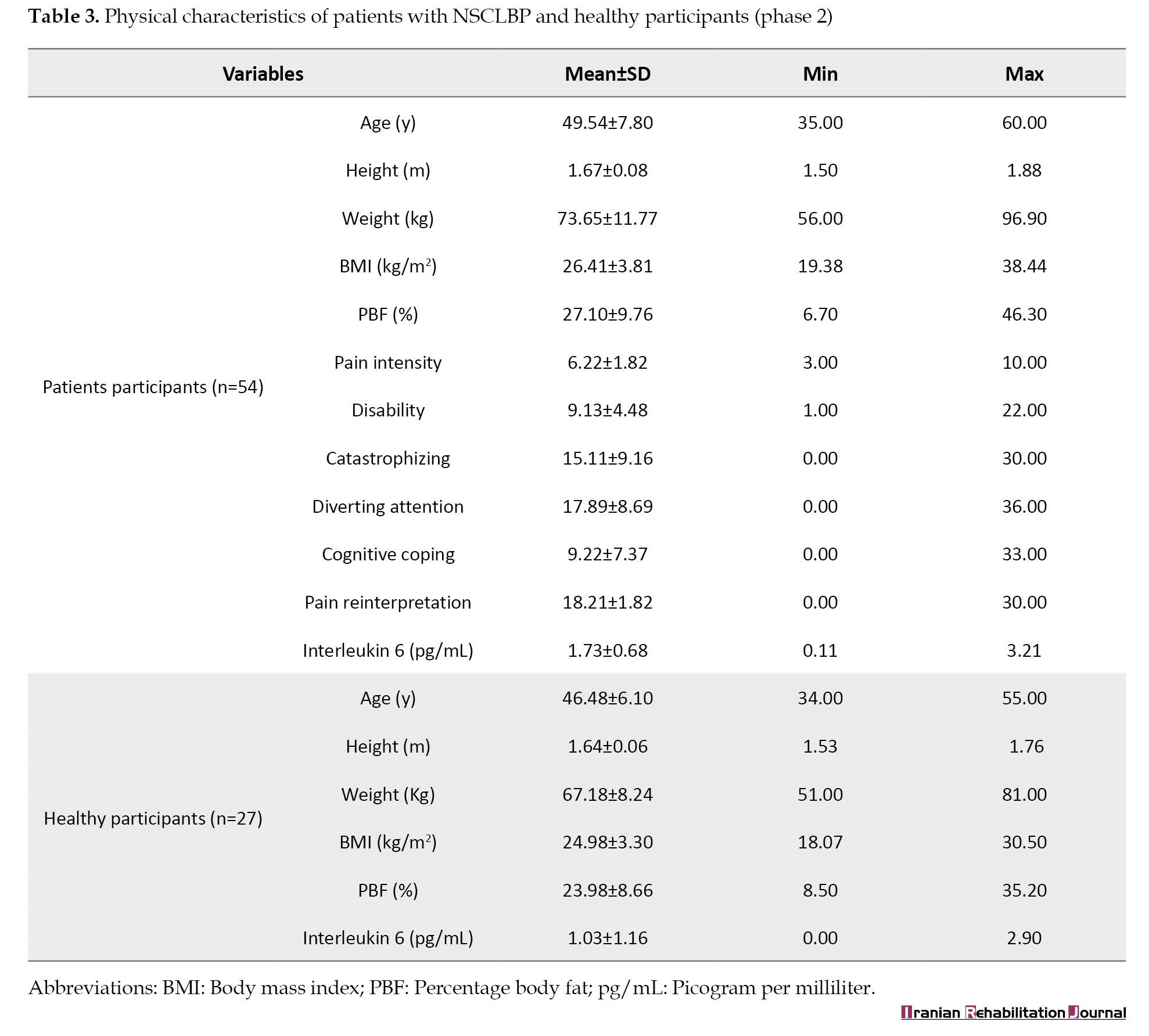
A total of 54 patients with NSCLBP (42.59% men, 57.41% women) and 27 healthy individuals (55.56% men, 44.44% women) participated in phase 2 of the trial. The mean age, height, weight, BMI, pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation of patients with NSCLBP were 49.54±7.80 years, 1.67±0.08 m, 73.65±11.77 kg, 26.41±3.81 kg/m2, 6.22±1.82, 9.13±4.48, 15.11±9.16, 17.89±8.69, 9.22±7.37, 18.21±1.82, and 1.73±0.68 pg/mL, respectively (Table 3).
Treatment groups (group 1 and 2) were comparable (P>0.05) in age, height, weight, BMI, percentage body fat, and baseline IL-6 concentrations, while patients in group 2 were significantly (P<0.05) higher in IL-6 concentration than the healthy participants (group 3) (Table 4 and Table 5).


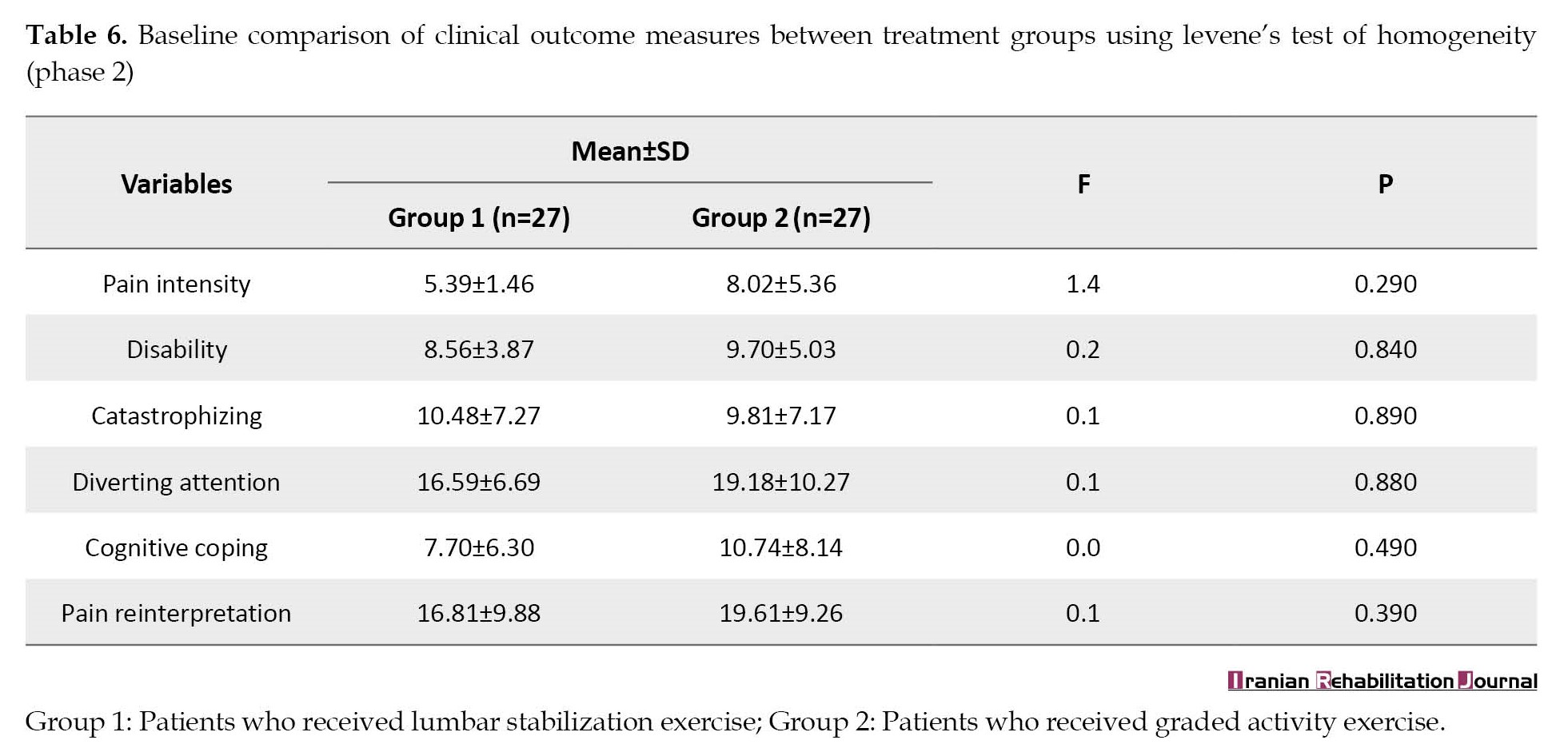
Also, treatment groups were comparable in baseline pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation (P>0.05) (Table 6).
Furthermore, this study showed a statistically significant increase (P<0.05) in IL-6 concentration after 10 weeks of LSE and GAE in patients with NSCLBP (Table 7).

The increase in IL-6 concentrations after LSE and GAE at 5 and 10 weeks, respectively, was statistically significant (P<0.05) (Table 8).

Also, this study showed a statistically significant decrease (P<0.05) in pain intensity, disability, and catastrophizing in patients with NSCLBP after 10 weeks of LSE, while diverting attention, cognitive coping, and reinterpretation of pain were comparable (P>0.05) (Table 9).

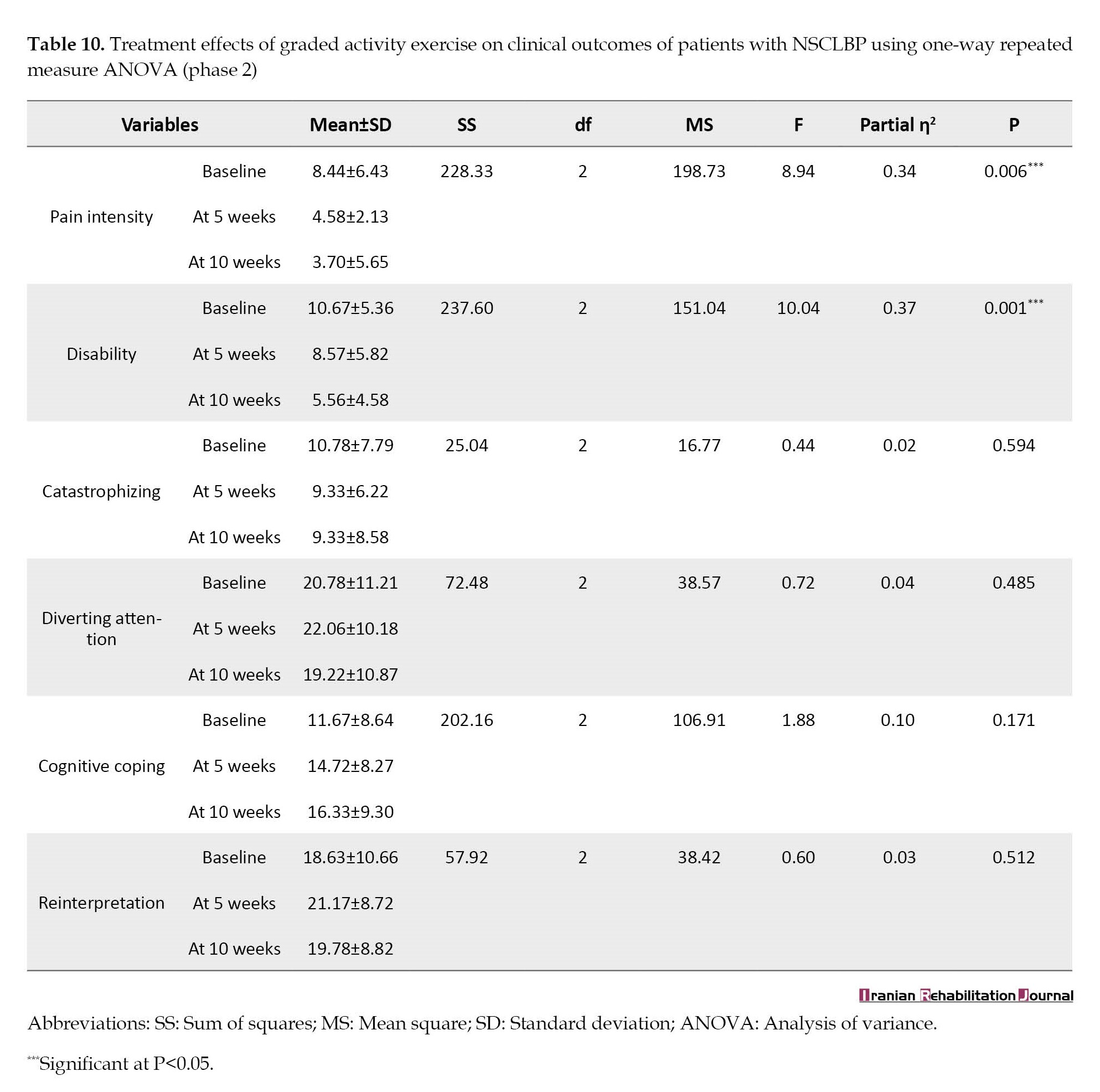
On the other hand, this study showed a statistically significant decrease (P<0.05) in pain intensity and disability in patients with NSCLBP after 10 weeks of GAE, while diverting attention, catastrophizing, cognitive coping, and reinterpretation of pain were comparable (P>0.05) (Table 10).
The decrease in pain intensity, disability, and catastrophizing was statistically significant (P<0.05) at 5 and 10 weeks of LSE, while the decrease in pain intensity, and disability after GAE was statistically significant (P<0.05) at 10 weeks only (Table 11).

LSE and GAE were comparable (P>0.05) in IL-6 concentrations (Table 12).

Also, LSE and GAE were comparable (P>0.05) in patients’ pain intensity, disability, diverting attention, cognitive coping, and pain reinterpretation with the difference that patients’ ability to catastrophize was significantly decreased (P<0.05) at 5 and 10 weeks of LSE compared to GAE (Table 13).

Discussion
This study evaluated the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, IL-6, COX2 and pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation in patients with NSCLBP. Phase 1 of the trial determined the responsiveness of concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 to LSE and GAE for 10 weeks. It was found that only the concentration of IL-6 was responsive to LSE and GAE, while concentrations of IL1A, IL18R1, IL18RAP, and COX2 were not responsive to LSE and GAE for 10 weeks. Hence, the trial on the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, and COX2 was stopped after phase 1, while the trial on the effects of LSE and GAE on concentrations of IL-6 continued in phase 2. For pain biomarker studies, Bayes’ theorem provides a useful guide for new studies because not all pain biomarkers may have predictive value [38]. Therefore, it is advisable to conduct an interim analysis for biomarkers in a prospective study, especially when limited data support such biomarkers.
The results of this study regarding patients’ mean age of 49.54 years are consistent with the reports that the prevalence of low back pain peaks between the mid-30s and mid-50s [39]. A similar result was reported in phase 2 with a significant concentration of IL-6 in the patients than healthy individuals in phase 1 of the study [25]. One argument in support of higher concentrations of IL-6 in patients with NSCLBP than in healthy control is its pro-inflammatory and anti-inflammatory roles in disease conditions [40, 41].
IL-6 concentrations increased after 10 weeks of LSE and GAE. The result of increased IL-6 concentration after 10 weeks of LSE is in contrast to the report of Capossela et al. [42]. Capossela et al. reported lower concentrations of IL-6 in patients with CLBP after long-term conservative treatments; although, the physiotherapy components of the conservative management were not classified [42]. Nevertheless, this result of increased IL-6 concentration in this present study is consistent with the report of increased levels of IL-6 following exercise [24, 40, 43, 44]. Legard and Pederson assert that IL-6 is mainly produced and released by contracting skeletal muscles, and IL-6 increases exponentially in proportion to the length of exercise and the amount of muscle mass engaged in the exercise [43]. Likewise, increased IL-6 concentration was reported after drug therapy involving tocilizumab for lumbar pain [45].
In this regard, Minobes-Molina et al. found that LSE increases IL-6 concentrations in patients with NSCLBP [24]. However, Minobes-Molina et al. reported that traditional exercise therapy decreases IL-6 in patients with NSCLBP [24]. However, it is worth noting that the interventional approach of this present study differs from that of Minobes-Molina et al. The present study administered only exercise therapies (LSE and GAE), while Minobes-Molina et al. used a multimodal approach, including transcutaneous electrical nerve stimulation, infrared therapy, and exercise therapies [24]. Also, exercise therapies in Minobes-Molina et al. were administered with moderate intensities (10 repetitions), while exercise therapies in this present study were administered with moderate and high intensities (10 and 15 repetitions) [24].
Even though IL-6 is a pro-inflammatory cytokine, studies have shown that the contracting skeletal muscle can produce IL-6 to mediate anti-inflammation [24, 40, 43, 46]. IL-6 has two signaling pathways termed trans-signaling and classic signaling [41]. IL-6 trans-signaling mediates pro-inflammatory effect, while IL-6 classical signaling mediates anti-inflammation and regenerative effects [41]. Classical signaling of IL-6 occurs through the activation of cell membrane-bound IL-6 receptors [41]. Thus, it can be concluded that the higher concentration of IL-6 observed in patients with NSCLBP at baseline in this study may be due to the pro-inflammatory effects of IL-6, while the increase in IL-6 concentrations after LSE and GAE in patients with NSCLBP may be due to the anti-inflammatory effects of IL-6 activated by the contraction of the skeletal muscles.
In summary, IL-6 is reported to mediate anti-inflammatory effects and plays a role in myogenesis in a classical signaling phenomenon [24, 40, 41, 43]. Collaboratively, LSE and GAE are reported to alleviate pain and increase muscle strength in patients with NSCLBP [6, 7, 8, 9, 10, 11, 30]. Thus, the result of this study on increased IL-6 concentration after LSE and GAE may help to explain the biochemical means of LSE and GAE to reduce pain intensity in patients with NSCLBP.
However, the effects of LSE and GAE on IL-6 concentrations were not significantly different in this study. This result that no significant difference is observed in IL-6 concentration between LSE and GAE in patients with NSCLBP is consistent with Legard and Pederson’s report [43]. Legard and Pederson opined that exercise-induced levels of cytokines depend on the intensity, mode, and frequency of the exercise [43]. Given that the 5 and 10 weeks of LSE and GAE differed in intensity and mode but not frequency, it is reasonable to imply that the exercise factor of frequency may be a crucial indicator for IL-6 response to exercise. In other words, the effects of LSE and GAE of different modes and intensities but with the same frequency on IL-6 concentrations in patients with NSCLBP are not significantly different. Sokunbi et al. found no significant difference between the two modes of exercise but found a significant difference between exercise frequencies in serotonin concentration in patients with CLBP [23].
Again, the results of this study on reduced pain intensity and disability after LSE and GAE are consistent with the reports of previous studies [6, 7, 8, 9, 10, 11, 30]. These results of reduction in pain intensity and disability after LSE and GAE imply that both LSE and GAE are effective in reducing pain and disability in patients with NSCLBP. However, LSE had a better therapeutic outlook than GAE because patient catastrophizing was significantly reduced only after 10 weeks of LSE. Harland and Martins reported that variability in catastrophizing scores over time is one of the factors suggesting effective treatment [47]. Also, this deduction of a more beneficial effect of LSE than GAE is buttressed by the result of this present study that the patient’s ability to catastrophize was significantly lower in LSE compared to GAE. Previous studies have highlighted the prognostic indication of pain catastrophizing in CLBP management [48, 49]. LSE may be more beneficial than GAE for treating patients with NSCLBP because LSE reduces catastrophizing in addition to reducing pain and disability in patients with NSCLBP.
Conclusion
LSE increases IL-6 concentrations in patients with NSCLBP while reducing pain, disability, and catastrophizing, while GAE increases IL-6 concentrations while reducing pain, and disability in patients with NSCLBP. Patients’ concentrations of IL1A, IL18R1, IL18RAP, and COX2 were not responsive to LSE and GAE. Both LSE and GAE were similar in effects on IL-6 concentrations, pain, and disability in patients with NSCLBP. Patients catastrophize less with LSE compared to GAE, hence suggesting more beneficial effects of LSE for patients with NSCLBP than GAE.
Strengths and weakness of this study
This study provides the clinical implications of LSE and GAE administration on the concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 in patients with NSCLBP. The results of this study may be useful as an objective means for clinicians and patients to evaluate the biochemical effects of LSE and GAE administrations in patients with NSCLBP. One limitation of this study is that little literature relatively supports the evaluation of the selected biochemical mediators in patients with NSCLBP for therapeutic exercises in clinical settings. Another limitation was that the investigators were unaware of the need to evaluate membrane-bound IL-6 receptors as part of the selected biochemical mediators given that IL-6 anti-inflammatory and myogenic roles rely on classical signaling through the membrane-bound IL-6 receptor. Hence, IL-6 responses to LSE and GAE in this study were interpreted by the effects of LSE and GAE on pain intensity and disability in patients with NSCLBP.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was obtained from the Health Research and Ethics Committee of an Academic Teaching Hospital (Code: ADM/DCST/HREC/APP/2638), National Orthopaedic Hospital, (Code: OH/90/C/IX), and a General Hospital (Code: LSHSC/2222/VOL.X/205). Also, participants’ consent was sought and obtained.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to all the participants of the study, especially those whose gave consent for their photographs to be used for illustration of the exercises. Also, the authors are grateful to all the staff of the Centre for Human and Zoonotic Virology (CHAZVY), College of Medicine, University of Lagos for their selfless efforts in producing the enzyme linked immunosorbent assay of all the biochemical mediators of this study.
References
Non-specific chronic low back pain (NSCLBP) represents the vast majority of chronic low back pain (CLBP) complaints consulted by physiotherapists [1]. It is defined as CLBP without a definite and recognizable primary disease, persisting for at least three months with clinical signs of muscle tension or stiffness localized below the costal margin of the spine and above the inferior gluteal folds in the presence or absence of sciatica [1]. The impact of NSCLBP on individuals and society is enormous because studies showed that it results in significant health burden, disability, absence from work, high cost of management, reduced quality of life, and increased psychological variables of anxiety with dissatisfaction and hopelessness [1-4].
Moreover, several treatment approaches for NSCLBP have evolved with differing principles at the centre of treatment algorithms [1, 2]. Therapeutic exercises are the first line of choice among different treatments for NSCLBP [1, 5]. Exercise therapy is effective for the management of NSCLBP resulting in improved clinical outcomes of pain, disability, and quality of life [1, 5, 6]. Also, among exercise therapies for NSCLBP, lumbar stabilization exercise (LSE) and graded activity exercises (GAE) are reported to be effective in reducing pain and disability [6]. LSE gained significant interest among CLBP researchers following the evidence that it activates the deep trunk muscles and restores synergic actions of the deep and superficial trunk muscles while reducing pain and disability in patients with NSCLBP [7, 8]. On the other hand, graded activity exercise addresses pain associated with fear of movement, unhelpful beliefs, and behavioral adaptations of NSCLBP while restoring dysfunctional muscle strength, endurance, and balance [9]. Interventionally, LSE emphasizes core stabilization using progressing strength and endurance exercises, while GAE uses behavioral quotas, pacing, and positive reinforcement as psychological constructs to enhance the muscle strength, endurance, and posture of patients with NSCLBP [9, 10, 11].
However, further studies on LSE and GAE in patients with NSCLBP are necessary because reports implicate numerous biochemical mediators in patients with NSCLBP [12-14]. Emerging evidence of pain mechanisms in NSCLBP suggests the need to profile biochemical mediators of pain for optimum intervention [3, 12]. Biochemically, the pain response is associated with the presence of inflammatory mediators of bradykinin, serotonin, histamine, adenosine triphosphate, prostaglandins, nitric oxides, cytokines, leukotrienes, cyclooxygenases, and neurotrophins [15]. Elevated levels of some biochemical mediators of pain have been reported in patients with NSCLBP [13, 16, 17, 18]. The cellular potentiation of some biochemical mediators is reported to influence the persistence of chronic pain, such as NSCLBP [18]. Also, studies support the evaluation of biochemical mediators of interleukin (IL)-6, tumor necrosis factor-alpha, interleukin 1A (IL1A), interleukin 18 receptor 1 (IL18R1), interleukin 18 receptor accessory protein (IL18RAP), cyclooxygenase 2 (COX2) and matrix metalloprotease 3 given their association with disc degeneration, pain intensity, and disability in patients with NSCLBP [16, 17, 19, 20].
Consequently, evaluating biochemical mediators of pain as part of treatment outcome is desirable since recommendations are presented for favourable treatments in NSCLBP. When implemented as part of treatment outcome, the results from such studies help broaden and refine the clinical significance of recommended treatments. Along with issues of exercise superiority [21], few studies examine the effects of therapeutic exercises on biochemical mediators in patients with CLBP with promising results. The available evidence suggests the state of inducible immune activation after therapeutic exercises in patients with LBP [21, 22, 23, 24]. Al-Obaidi and Mahmoud studied McKenzie’s exercise on immune response in acute LBP [23], while Sokunbi et al. examined the effects of LSE on serotonin in CLBP [22]. Minobes-Molina et al. investigated the effect of multimodal treatments, including exercise therapies on IL-6 concentrations in NSCLBP [24]. Thus, few or no studies examine the effects of only therapeutic exercise on immune response in NSCLBP. Therefore, this study was designed to evaluate the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, IL6 COX2 and clinical outcomes of pain intensity, disability, catastrophizing, diverting attention, cognitive coping with pain reinterpretation in patients with NSCLBP. This study hypothesized that the effects of LSE and GAE on concentrations of selected cytokines and clinical outcomes are not significantly different.
Materials and Methods
Study design
The study was a single-blind parallel trial with an adaptive trial design. The study was registered with the Pan African Clinical Trial Registry. The trial was conducted in two phases, before which a feasibility study was conducted. Phase 1 obtained the baseline, age, and gender values of IL1A, IL18R1, IL18RAP, IL-6, and COX2 in patients with NSCLBP as reported by Oghumu et al. [25]. Also, phase 1 ascertained the responsiveness of IL1A, IL18R1, IL18RAP, IL-6, and COX2 to LSE and GAE for 10 weeks in patients with NSCLBP using an interim analysis. Phase 2 evaluated the effects of LSE and GAE on the responsive biochemical mediator (s) and the selected clinical outcomes in patients with NSCLBP.
Sampling technique and selection criteria
Consecutive sampling was used to recruit all participants. Participants’ selection criteria were hinged on commonly reported domains for inclusion criteria in NSCLBP studies and the mean half-life of commonly used analgesics [26, 27]. The inclusion criteria included patients referred for only NSCLBP with or without radiating symptoms of at least three months, patients having no other site of pain, patients aged 18 to 60 years, and healthy individuals with no history of LBP in the last six months. The exclusion criteria included patients with a diagnosis of spinal inflammatory disease, such as ankylosing spondylitis, a history of spinal fracture or dislocation, motor or sensory deficit, pregnancy, any systemic or medical condition, such as diabetes, hematological disorder, acute or chronic liver diseases, autoimmune disease, use of oral or topical pain medications (non-steroidal anti-inflammatory drugs, steroid or any form of analgesics within the last two days before presentation), intention not to stop the analgesics during the study, a history of psychotropic medications, such as benzodiazepine, and open wound in any part of the body. Patients and healthy participants with a history of smoking and drinking alcohol in the last six months were excluded from the study.
Randomization, study groups, and blinding of participants
Patients with NSCLBP were randomly allocated to LSE and GAE by computer-generated block sizes varying between 4 and 8. Three groups existed in each phase of the trial. Group 1 was patients who received LSE, Group 2 was patients who received GAE, and Group 3 was healthy participants who received no treatment. Treatment appointments were scheduled for patients on different days to ensure blinding.
Determination of sample size
Oghumu et al. reported that in phase 1, 16 patients with NSCLBP and 16 healthy participants were calculated [25]. Each treatment group in phase 1 had 8 patients and 6 patients completed the treatments. For phase 2, Chan’s formula (Equation 1) was used, which assumed a type 1 error and a power of 80% [28].
1. n=c×π1(1-π1)+π2(1-π2)/(π1-π2)2
Given a two-sided test of 5%, the formula assumes that a successful outcome of 25% in one intervention will only be relevant if we observe a 40% effect size of absolute improvement in the other intervention. Therefore, given that n=sample size, π1=0.25, π2=0.65, c depends on the power of the sample (c=7.9 for a power of 80%), n=7.9×0.25(1-0.25)+0.65(1-0.65)/(0.25-0.65)2=21. For three groups, n=3×21=63. Assuming a 20% drop-out, n=63×1.25=78.75. Hence, 54 eligible patients with NSCLBP were randomly assigned to the treatment groups (Figure 1). Also, 27 volunteered healthy participants were recruited as control for baseline values.
Procedure for data collection
The same procedure was employed for the two phases of the trial. Participants’ history and physical assessment were performed by the researcher to ascertain the inclusion and exclusion criteria. Then participants were given commencement dates for the study. On the first day of the participants’ arrival, 8.5 mL of blood was drawn from their right arm by a phlebotomist into test tubes containing ethylenediaminetetraacetic acid as described by Vaught and Henderson [29]. All blood samples were taken at 11 AM. The test tubes were preserved at +4°C in a container filled with cold packs and transported to a central research laboratory where the blood samples were processed and stored at -80°C for analysis.
Participants’ age and gender were obtained, heights were measured in meters (m) with the health-0-meter incorporated, Bridgeview, Illinois, United States of America, weights in kilograms (kg) with the Hana scale (model BR-9011-Germany), and percentage body fats in % with the Omron BF306 monitor. The body mass index (BMI) of participants was estimated in kg/m2. Also, patients’ clinical outcomes were assessed with the following instruments, pain intensity with a visual analogue scale (VAS), disability with a Roland Morris disability questionnaire 24 (RMDQ24), catastrophizing, diverting attention, cognitive coping, and pain reinterpretation with a coping strategy questionnaire 24 (CSQ24), respectively.
Treatments were administered two times a week for 10 weeks of 20 sessions. Post-treatment concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 were assessed on the first day, fifth and tenth weeks, respectively. Also, post-treatment pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation were assessed in the fifth and tenth weeks, respectively.
Treatment procedures
The principles of exercise prescription were followed for LSE and GAE. Stretching exercises were performed before the LSE and GAE. Stretching was only indicated for observed tight muscles. A session of LSE or GAE lasted at least 45 minutes at three sets of repetitions. The abdominal drawing-in maneuver (Figure 2) was incorporated in all phases of the LSE, while cognitive behavioural therapy and bicycle ergometry were used in all phases of GAE (Figure 3). Phase 1 of the trial was conducted for 7 months, while phase 2 was conducted for 11 months.
Stretching exercises
Stretching exercises were performed on tight iliopsoas, rectus femoris, piriformis, and hamstrings of the lower extremities. Stretches were held for 30 s and repeated thrice.
Lumbar stabilization exercise (LSE)
LSE was based on the treatment program described in a previous study [30]. Three phases of the LSE program existed (Appendix). Phase 1 of the LSE was conducted for one session and it involved contraction of the transversus abdominis, multifidus, pelvic floor muscles, and diaphragm. It also involved isometric contraction of the erector spinalis and gluteus maximus in prone lying. Phase 2 LSE was conducted for 5 weeks and consisted of moderate-intensity (10 repetitions) closed-chain isometric strengthening of the gluteus maximus in prone, gluteus medius and minimus in side-lying, adductor magnus and brevis, iliopsoas in high sitting, quadriceps in supine and bridging exercise. It also involved strengthening exercises in functional position on all fours, full and semi-squatting, and proprioception training. Phase 3 of LSE was conducted for 5 weeks and consisted of high-intensity exercises (15 repetitions) in open-chain involving isometric strengthening of the same muscles in phase 2. It also involved isometric strengthening in open-chain functional positions on all fours, full and semi-squatting, and proprioception training.
Graded activity exercise (GAE)
GAE was based on the treatment program described by previous studies [9, 30]. The GAE aimed to increase patients’ activity tolerance using individualized and sub-maximal exercises with cognitive behavioral principles, such as ignoring pain, pacing, explaining pain mechanisms, and reinforcing wellness behavior of exercise benefits. The individualized and sub-maximal exercises included isometric strengthening exercises to the quadriceps femoris, hamstring, gluteus maximus, gluteus medius and minimus, erector spinae, abdominal muscles, and bicycle ergometry (Appendix). The GAE was performed in three phases of sub-maximal exercises. Phases 1 and 2 were moderate-intensity exercises (10 repetitions) with 2 kg weights, while phase 3 consisted of high intensity (15 repetitions) with 3 kg weights. Phase 1 of the GAE lasted for one session, while phases 2 and 3 lasted for 5 weeks each.
Outcome measures
Visual analogue scale (VAS)
The VAS was represented by a 10 cm horizontal line. The horizontal line is anchored with no pain at one end and very extreme pain at the other end [31]. Patients were instructed to indicate their pain intensity by placing a vertical line on the horizontal line. The VAS is reported to have high reliability and validity scores [31].
Roland morris disability questionnaire 24 (RMDQ24)
The RMDQ24 has 24 items on physical functions likely to be affected by low back pain [32]. Its score involves adding up the number of items checked. The scores ranged from 0 (no disability) to 24 (maximum disability) [32]. The RMDQ24 has good construct validity with Cronbach’s scores ranging from 0.7 to 0.90 [32].
Coping strategy questionnaire 24 (CSQ24)
The CSQ24 consists of 23 items about coping strategies [33]. It has a 7-point Likert scale ranging from “never do that” and “always do that,” and one item measuring perceived control over pain with a 7-point Likert scale ranging from “no control” and “complete control [33].” It has four subscales, catastrophizing, diverting attention, reinterpreting, and cognitive coping. Each subscale is scored from zero to 36. The CSQ24 has a good level of validity [33].
Enzyme-linked immunosorbent assay (ELISA)
The ELISA was used to quantify the concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 in the collected blood samples, as described by Chiswick et al. [34]. The ELISA follows the same procedure, as described by Oghumu et al. [25] in phase 1 of the trial. The ELISA is very sensitive and valid to detect and measure proteins on the pictogram scale [35].
Data analysis
Data were analyzed using IBM SPSS software, Version 26. Data for concentrations of IL1A, IL18R1, IL-6, IL18RAP, and COX2 were log-transformed as recommended by the US center for disease control and prevention [36]. Descriptive statistics summarized the Mean±SD and percentages of the data. The Bayesian one-way repeated measure analysis of variance (ANOVA) was used to evaluate the responsiveness of the concentrations of IL1A, IL18R1, IL6, IL18RAP, and COX2 to LSE and GAE in phase 1 of the trial. The previously stated evidential categories of Bayes factor (BF10) reported in the literature were used to inform the decision to respond [37].
Inferential statistics of Kruskal-Wallis one-way ANOVA tested the significant difference among study groups. The Mann-Whitney U test tested the significant difference in IL-6 concentrations between study groups. An independent t-test was used to test the significant difference in baseline values of clinical outcomes between treatment groups. The Friedman test determined the effects of LSE and GAE on patients’ concentrations of IL-6. Also, the Wilcoxon test determined the within-group comparison of treatment effects of LSE and GAE on patients’ concentrations of IL-6. One-way repeated measure ANOVA determined the treatment effects of LSE and GAE on patients’ pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation. The 95% CI was used for mean differences. The significance level was set at P<0.05.
Results
Sixteen patients with NSCLBP (56.25% men and 43.75% women) and 14 healthy individuals (50% men and 50% women) participated in phase 1 of the trial. Table 1 and Table 2 present the response of concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 to LSE and GAE in phase 1 of the trial, respectively.


The result revealed that the concentrations of IL1A, IL18R1, IL18RAP, and COX2 were not responsive to LSE and GAE (BF10<1) after 10 weeks of treatments, while the concentrations of IL-6 were responsive (BF10>1) (Table 1 and Table 2). Hence, the trial on the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, and COX2 was stopped, while the trial on the effects of LSE and GAE on concentrations of IL-6 continued in phase 2.
A total of 54 patients with NSCLBP (42.59% men, 57.41% women) and 27 healthy individuals (55.56% men, 44.44% women) participated in phase 2 of the trial. The mean age, height, weight, BMI, pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation of patients with NSCLBP were 49.54±7.80 years, 1.67±0.08 m, 73.65±11.77 kg, 26.41±3.81 kg/m2, 6.22±1.82, 9.13±4.48, 15.11±9.16, 17.89±8.69, 9.22±7.37, 18.21±1.82, and 1.73±0.68 pg/mL, respectively (Table 3).

Treatment groups (group 1 and 2) were comparable (P>0.05) in age, height, weight, BMI, percentage body fat, and baseline IL-6 concentrations, while patients in group 2 were significantly (P<0.05) higher in IL-6 concentration than the healthy participants (group 3) (Table 4 and Table 5).


Also, treatment groups were comparable in baseline pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation (P>0.05) (Table 6).

Furthermore, this study showed a statistically significant increase (P<0.05) in IL-6 concentration after 10 weeks of LSE and GAE in patients with NSCLBP (Table 7).

The increase in IL-6 concentrations after LSE and GAE at 5 and 10 weeks, respectively, was statistically significant (P<0.05) (Table 8).

Also, this study showed a statistically significant decrease (P<0.05) in pain intensity, disability, and catastrophizing in patients with NSCLBP after 10 weeks of LSE, while diverting attention, cognitive coping, and reinterpretation of pain were comparable (P>0.05) (Table 9).

On the other hand, this study showed a statistically significant decrease (P<0.05) in pain intensity and disability in patients with NSCLBP after 10 weeks of GAE, while diverting attention, catastrophizing, cognitive coping, and reinterpretation of pain were comparable (P>0.05) (Table 10).

The decrease in pain intensity, disability, and catastrophizing was statistically significant (P<0.05) at 5 and 10 weeks of LSE, while the decrease in pain intensity, and disability after GAE was statistically significant (P<0.05) at 10 weeks only (Table 11).

LSE and GAE were comparable (P>0.05) in IL-6 concentrations (Table 12).

Also, LSE and GAE were comparable (P>0.05) in patients’ pain intensity, disability, diverting attention, cognitive coping, and pain reinterpretation with the difference that patients’ ability to catastrophize was significantly decreased (P<0.05) at 5 and 10 weeks of LSE compared to GAE (Table 13).

Discussion
This study evaluated the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, IL-6, COX2 and pain intensity, disability, catastrophizing, diverting attention, cognitive coping, and pain reinterpretation in patients with NSCLBP. Phase 1 of the trial determined the responsiveness of concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 to LSE and GAE for 10 weeks. It was found that only the concentration of IL-6 was responsive to LSE and GAE, while concentrations of IL1A, IL18R1, IL18RAP, and COX2 were not responsive to LSE and GAE for 10 weeks. Hence, the trial on the effects of LSE and GAE on concentrations of IL1A, IL18R1, IL18RAP, and COX2 was stopped after phase 1, while the trial on the effects of LSE and GAE on concentrations of IL-6 continued in phase 2. For pain biomarker studies, Bayes’ theorem provides a useful guide for new studies because not all pain biomarkers may have predictive value [38]. Therefore, it is advisable to conduct an interim analysis for biomarkers in a prospective study, especially when limited data support such biomarkers.
The results of this study regarding patients’ mean age of 49.54 years are consistent with the reports that the prevalence of low back pain peaks between the mid-30s and mid-50s [39]. A similar result was reported in phase 2 with a significant concentration of IL-6 in the patients than healthy individuals in phase 1 of the study [25]. One argument in support of higher concentrations of IL-6 in patients with NSCLBP than in healthy control is its pro-inflammatory and anti-inflammatory roles in disease conditions [40, 41].
IL-6 concentrations increased after 10 weeks of LSE and GAE. The result of increased IL-6 concentration after 10 weeks of LSE is in contrast to the report of Capossela et al. [42]. Capossela et al. reported lower concentrations of IL-6 in patients with CLBP after long-term conservative treatments; although, the physiotherapy components of the conservative management were not classified [42]. Nevertheless, this result of increased IL-6 concentration in this present study is consistent with the report of increased levels of IL-6 following exercise [24, 40, 43, 44]. Legard and Pederson assert that IL-6 is mainly produced and released by contracting skeletal muscles, and IL-6 increases exponentially in proportion to the length of exercise and the amount of muscle mass engaged in the exercise [43]. Likewise, increased IL-6 concentration was reported after drug therapy involving tocilizumab for lumbar pain [45].
In this regard, Minobes-Molina et al. found that LSE increases IL-6 concentrations in patients with NSCLBP [24]. However, Minobes-Molina et al. reported that traditional exercise therapy decreases IL-6 in patients with NSCLBP [24]. However, it is worth noting that the interventional approach of this present study differs from that of Minobes-Molina et al. The present study administered only exercise therapies (LSE and GAE), while Minobes-Molina et al. used a multimodal approach, including transcutaneous electrical nerve stimulation, infrared therapy, and exercise therapies [24]. Also, exercise therapies in Minobes-Molina et al. were administered with moderate intensities (10 repetitions), while exercise therapies in this present study were administered with moderate and high intensities (10 and 15 repetitions) [24].
Even though IL-6 is a pro-inflammatory cytokine, studies have shown that the contracting skeletal muscle can produce IL-6 to mediate anti-inflammation [24, 40, 43, 46]. IL-6 has two signaling pathways termed trans-signaling and classic signaling [41]. IL-6 trans-signaling mediates pro-inflammatory effect, while IL-6 classical signaling mediates anti-inflammation and regenerative effects [41]. Classical signaling of IL-6 occurs through the activation of cell membrane-bound IL-6 receptors [41]. Thus, it can be concluded that the higher concentration of IL-6 observed in patients with NSCLBP at baseline in this study may be due to the pro-inflammatory effects of IL-6, while the increase in IL-6 concentrations after LSE and GAE in patients with NSCLBP may be due to the anti-inflammatory effects of IL-6 activated by the contraction of the skeletal muscles.
In summary, IL-6 is reported to mediate anti-inflammatory effects and plays a role in myogenesis in a classical signaling phenomenon [24, 40, 41, 43]. Collaboratively, LSE and GAE are reported to alleviate pain and increase muscle strength in patients with NSCLBP [6, 7, 8, 9, 10, 11, 30]. Thus, the result of this study on increased IL-6 concentration after LSE and GAE may help to explain the biochemical means of LSE and GAE to reduce pain intensity in patients with NSCLBP.
However, the effects of LSE and GAE on IL-6 concentrations were not significantly different in this study. This result that no significant difference is observed in IL-6 concentration between LSE and GAE in patients with NSCLBP is consistent with Legard and Pederson’s report [43]. Legard and Pederson opined that exercise-induced levels of cytokines depend on the intensity, mode, and frequency of the exercise [43]. Given that the 5 and 10 weeks of LSE and GAE differed in intensity and mode but not frequency, it is reasonable to imply that the exercise factor of frequency may be a crucial indicator for IL-6 response to exercise. In other words, the effects of LSE and GAE of different modes and intensities but with the same frequency on IL-6 concentrations in patients with NSCLBP are not significantly different. Sokunbi et al. found no significant difference between the two modes of exercise but found a significant difference between exercise frequencies in serotonin concentration in patients with CLBP [23].
Again, the results of this study on reduced pain intensity and disability after LSE and GAE are consistent with the reports of previous studies [6, 7, 8, 9, 10, 11, 30]. These results of reduction in pain intensity and disability after LSE and GAE imply that both LSE and GAE are effective in reducing pain and disability in patients with NSCLBP. However, LSE had a better therapeutic outlook than GAE because patient catastrophizing was significantly reduced only after 10 weeks of LSE. Harland and Martins reported that variability in catastrophizing scores over time is one of the factors suggesting effective treatment [47]. Also, this deduction of a more beneficial effect of LSE than GAE is buttressed by the result of this present study that the patient’s ability to catastrophize was significantly lower in LSE compared to GAE. Previous studies have highlighted the prognostic indication of pain catastrophizing in CLBP management [48, 49]. LSE may be more beneficial than GAE for treating patients with NSCLBP because LSE reduces catastrophizing in addition to reducing pain and disability in patients with NSCLBP.
Conclusion
LSE increases IL-6 concentrations in patients with NSCLBP while reducing pain, disability, and catastrophizing, while GAE increases IL-6 concentrations while reducing pain, and disability in patients with NSCLBP. Patients’ concentrations of IL1A, IL18R1, IL18RAP, and COX2 were not responsive to LSE and GAE. Both LSE and GAE were similar in effects on IL-6 concentrations, pain, and disability in patients with NSCLBP. Patients catastrophize less with LSE compared to GAE, hence suggesting more beneficial effects of LSE for patients with NSCLBP than GAE.
Strengths and weakness of this study
This study provides the clinical implications of LSE and GAE administration on the concentrations of IL1A, IL18R1, IL18RAP, IL-6, and COX2 in patients with NSCLBP. The results of this study may be useful as an objective means for clinicians and patients to evaluate the biochemical effects of LSE and GAE administrations in patients with NSCLBP. One limitation of this study is that little literature relatively supports the evaluation of the selected biochemical mediators in patients with NSCLBP for therapeutic exercises in clinical settings. Another limitation was that the investigators were unaware of the need to evaluate membrane-bound IL-6 receptors as part of the selected biochemical mediators given that IL-6 anti-inflammatory and myogenic roles rely on classical signaling through the membrane-bound IL-6 receptor. Hence, IL-6 responses to LSE and GAE in this study were interpreted by the effects of LSE and GAE on pain intensity and disability in patients with NSCLBP.
Ethical Considerations
Compliance with ethical guidelines
Ethical approval was obtained from the Health Research and Ethics Committee of an Academic Teaching Hospital (Code: ADM/DCST/HREC/APP/2638), National Orthopaedic Hospital, (Code: OH/90/C/IX), and a General Hospital (Code: LSHSC/2222/VOL.X/205). Also, participants’ consent was sought and obtained.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to all the participants of the study, especially those whose gave consent for their photographs to be used for illustration of the exercises. Also, the authors are grateful to all the staff of the Centre for Human and Zoonotic Virology (CHAZVY), College of Medicine, University of Lagos for their selfless efforts in producing the enzyme linked immunosorbent assay of all the biochemical mediators of this study.
References
- Chiarotto A, Koes BW. Nonspecific low back pain. The New England Journal of Medicine. 2022; 386(18):1732-40. [DOI:10.1056/NEJMcp2032396] [PMID]
- Wong AYL, Karppinen J, Samartzis D. Low back pain in older adults: Risk factors, management options and future directions. Scoliosis and Spinal Disorders. 2017; 12:14. [DOI:10.1186/s13013-017-0121-3] [PMID]
- Suntsov V, Jovanovic F, Knezevic E, Candido KD, Knezevic NN. Can implementation of genetics and pharmacogenomics improve treatment of chronic low back pain? Pharmaceutics. 2020; 12(9):894. [DOI:10.3390/pharmaceutics12090894.] [PMID]
- Pergolizzi JV Jr, LeQuang JA. Rehabilitation for low back pain: a narrative review for managing pain and improving function in acute and chronic conditions. Pain and Therapy. 2020; 9(1):83-96. [DOI:10.1007/s40122-020-00149-5] [PMID]
- Hayden JA, Ellis J, Ogilvie R, Stewart SA, Bagg MK, Stanojevic S, et al. Some types of exercise are more effective than others in people with chronic low back pain: A network meta-analysis. Journal of Physiotherapy. 2021; 67(4):252-62. [DOI:10.1016/j.jphys.2021.09.004] [PMID]
- Hoffmann TC, Maher CG, Briffa T, Sherrington C, Bennell K, Alison J, et al. Prescribing exercise interventions for patients with chronic conditions. CMAJ. 2016; 188(7):510-8. [DOI:10.1503/cmaj.150684] [PMID]
- Kwon SH, Oh SJ, Kim DH. The effects of lumbar stabilization exercise on transversus abdominis muscle activation capacity and function in low back pain patients. Isokinet Exerc Sci. 2020; 28(2):147-52. [DOI:10.3233/IES-182127]
- Akodu AK, Akindutire OM. The effect of stabilization exercise on pain-related disability, sleep disturbance, and psychological status of patients with non-specific chronic low back pain. The Korean Journal of Pain. 2018; 31(3):199-205. [DOI:10.3344/kjp.2018.31.3.199] [PMID] [PMCID]
- Magalhães MO, Comachio J, Ferreira PH, Pappas E, Marques AP. Effectiveness of graded activity versus physiotherapy in patients with chronic nonspecific low back pain: midterm follow up results of a randomized controlled trial. Brazilian Journal of Physical Therapy. 2018; 22(1):82-91. [DOI:10.1016/j.bjpt.2017.07.002] [PMID] [PMCID]
- Laura G, Jones G. Effectiveness of graded exercise & graded exposure for chronic nonspecific low back pain: A rapid review. Physiotherapy. 2020; 107(Supplement 1):E102.[DOI:10.1016/j.physio.2020.03.145]
- Saragiotto BT, Maher CG, Yamato TP, Costa LO, Menezes Costa LC, Ostelo RW, et al. Motor control exercise for chronic non-specific low-back pain. The Cochrane Database of Systematic Reviews. 2016; 2016(1):CD012004. [DOI:10.1002/14651858.CD012004] [PMID] [PMCID]
- Ota Y, Connolly M, Srinivasan A, Kim J, Capizzano AA, Moritani T. Mechanisms and origins of spinal pain: from molecules to anatomy, with diagnostic clues and imaging findings. Radiographics. 2020; 40(4):1163-81. [DOI:10.1148/rg.2020190185] [PMID]
- Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific low back pain: Inflammatory profiles of patients with acute and chronic pain. The Clinical Journal of Pain. 2019; 35(10):818-25. [DOI:10.1097/AJP.0000000000000745] [PMID] [PMCID]
- Gawda P, Zawadka M, Dmoszyńska-Graniczka E. Selected physiotherapeutic techniques and immune response in low back pain. J Edu, Health & Sports. 2017; 7(4): 657-64. [Link]
- Omoigui S. The biochemical origin of pain--proposing a new law of pain: The origin of all pain is inflammation and the inflammatory response. Part 1 of 3--a unifying law of pain. Medical Hypotheses. 2007; 69(1):70-82. [DOI:10.1016/j.mehy.2006.11.028] [PMID] [PMCID]
- Weber KT, Alipui DO, Sison CP, Bloom O, Quraishi S, Overby MC, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Research & Therapy. 2016; 18:3. [DOI:10.1186/s13075-015-0887-8] [PMID] [PMCID]
- Lim YZ, Wang Y, Cicuttini FM, Hughes HJ, Chou L, Urquhart DM, et al. Association between inflammatory biomarkers and nonspecific low back pain: A systematic review. The Clinical Journal of Pain. 2020; 36(5):379-89. [DOI:10.1097/AJP.0000000000000810] [PMID]
- Linher-Melville K, Shah A, Singh G. Sex differences in neuro(auto)immunity and chronic sciatic nerve pain. Biology of Sex Differences. 2020; 11(1):62. [DOI:10.1186/s13293-020-00339-y] [PMID] [PMCID]
- Bjorland S, Moen A, Schistad E, Gjerstad J, Røe C. Genes associated with persistent lumbar radicular pain; a systematic review. BMC Musculoskeletal Disorders. 2016; 17(1):500. [DOI:10.1186/s12891-016-1356-5] [PMID] [PMCID]
- Omair A, Holden M, Lie BA, Reikeras O, Brox JI. Treatment outcome of chronic low back pain and radiographic lumbar disc degeneration are associated with inflammatory and matrix degrading gene variants: A prospective genetic association study. BMC Musculoskeletal Disorders. 2013; 14:105. [DOI:10.1186/1471-2474-14-105] [PMID] [PMCID]
- Owen PJ, Miller CT, Mundell NL, Verswijveren SJJM, Tagliaferri SD, Brisby H, et aL. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. British Journal of Sports Medicine. 2020; 54(21):1279-87. [DOI:10.1136/bjsports-2019-100886] [PMID] [PMCID]
- Al-Obaidi S, Mahmoud F. Immune responses following McKenzie lumbar spine exercise in individuals with acute low back pain: A preliminary study. Acta Medica Academica. 2014; 43(1):19-29. [DOI:10.5644/ama2006-124.96] [PMID]
- Sokunbi O, Watt P, Moore A. A randomized controlled trial (RCT) on the effects of frequency of application of spinal stabilisation exercises on plasma serotonin levels in participants with chronic low back pain. Physiotherapy Singapore. 2014; 11(2):9-16. [DOI:10.9790/0853-1310197101]
- Minobes-Molina E, Nogués MR, Giralt M, Casajuana C, de Souza DLB, Jerez-Roig J, et al. Effectiveness of specific stabilization exercise compared with traditional trunk exercise in women with non-specific low back pain: A pilot randomized controlled trial. PeerJ. 2020; 8:e10304. [DOI:10.7717/peerj.10304] [PMID] [PMCID]
- Oghumu SN, Tella BA, Okafor UAC, Salu OB. Baseline values of selected biochemical mediators of pain implicated in patients with non-specific chronic low back pain. Iranian Rehabilitation Journal. 2023; [Unpublished]. [Link]
- Amundsen PA, Evans DW, Rajendran D, Bright P, Bjørkli T, Eldridge S, et al. Inclusion and exclusion criteria used in non-specific low back pain trials: A review of randomised controlled trials published between 2006 and 2012. BMC Musculoskeletal Disorders. 2018 Apr 12; 19(1):113. [DOI:10.1186/s12891-018-2034-6] [PMID] [PMCID]
- Iolascon G, Giménez S, Mogyorósi D. A review of aceclofenac: Analgesic and anti-inflammatory effects on musculoskeletal disorders. Journal of Pain Research. 2021; 14:3651-63. [DOI:10.2147/JPR.S326101] [PMID] [PMCID]
- Chan YH. Randomised controlled trials (RCTs)--sample size: the magic number? Singapore Medical Journal. 2003; 44(4):172-4. [PMID]
- Vaught JB, Henderson MK. Biological sample collection, processing, storage and information management. IARC Scientific Publications. 2011; (163):23-42. [PMID]
- Macedo LG, Latimer J, Maher CG, Hodges PW, McAuley JH, Nicholas MK, et al. Effect of motor control exercises versus graded activity in patients with chronic nonspecific low back pain: A randomized controlled trial. Physical Therapy. 2012; 92(3):363-77. [DOI:10.2522/ptj.20110290] [PMID]
- Price DD, Staud S, Robinson ME. How should we use the visual analogue scale (VAS) in rehabilitation outcomes? In: Visual analogue scales as ratio scales: an alternative to the view of Kersten et al. J Rehabil Med. 2012; 44(9): 800-804. [DOI:10.2340/16501977-1031] [PMID] [PMCID]
- Roland M, Fairbank J. The roland-morris disability questionnaire and the oswestry disability questionnaire. Spine (Phila Pa 1976). 2000; 25(24):3115-24. [DOI:10.1097/00007632-200012150-00006] [PMID]
- Harland N, Ryan CG. The value of pain coping constructs in subcategorising back pain patients according to risk of poor outcome. BioMed Research International. 2013; 2013:898573.[DOI:10.1155/2013/898573] [PMID] [PMCID]
- Chiswick EL, Duffy E, Japp B, Remick D. Detection and quantification of cytokines and other biomarkers. Methods in Molecular Biology. 2012; 844:15-30. [DOI:10.1007/978-1-61779-527-5_2] [PMID] [PMCID]
- Konstantinou GN. Enzyme-linked immunosorbent assay (ELISA). Methods in Molecular Biology. 2017; 1592:79-94.[DOI:10.1007/978-1-4939-6925-8_7] [PMID]
- Centers for Disease Control and Prevention. Wichita clinical study data access [Internet]. 2018 [Updated 2018 August 14]. Available from: [Link]
- Quintana DS, Williams DR. Bayesian alternatives for common null-hypothesis significance tests in psychiatry: A non-technical guide using JASP. BMC Psychiatry. 2018; 18(1):178. [DOI:10.1186/s12888-018-1761-4] [PMID] [PMCID]
- Robinson M, Boissoneault J, Sevel L, Letzen J, Staud R. The effect of base rate on the predictive value of brain biomarkers. The Journal of Pain. 2016; 17(6):637-41. [DOI:10.1016/j.jpain.2016.01.476] [PMID] [PMCID]
- Tella BA, Oghumu SN, Gbiri CAO. Efficacy of transcutaneous electrical nerve stimulation and interferential current on tactile acuity of individuals with nonspecific chronic low back pain. Neuromodulation. 2022; 25(8):1403-9. [DOI:10.1111/ner.13522] [PMID]
- Docherty S, Harley R, McAuley JJ, Crowe LAN, Pedret C, Kirwan PD, et al. The effect of exercise on cytokines: Implications for musculoskeletal health: A narrative review. BMC Sports Science, Medicine & Rehabilitation. 2022; 14(1):5. [DOI:10.1186/s13102-022-00397-2] [PMID] [PMCID]
- Rose-John S. Interleukin-6 signalling in health and disease. F1000Research. 2020; 9:F1000 Faculty Rev-1013 [DOI:10.12688/f1000research.26058.1] [PMID] [PMCID]
- Capossela S, Pavlicek D, Bertolo A, Landmann G, Stoyanov JV. Unexpectedly decreased plasma cytokines in patients with chronic back pain. Journal of Pain Research. 2018; 11:1191-8. [DOI:10.2147/JPR.S153872] [PMID] [PMCID]
- Legard GE, Pedersen BK. Muscle as an endocrine organ In: Zoladz JA, editor. Muscle and exercise physiology. Amsterdam: Elsevier; 2019. [DOI:10.1016/B978-0-12-814593-7.00013-X]
- Peake JM, Della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: Regulatory mechanisms and exercise effects. Exercise Immunology Review. 2015; 21:8-25. [PMID]
- Sainoh T, Orita S, Miyagi M, Suzuki-Narita M, Sakuma Y, Oikawa Y, et al. Improvements in intractable lumbar and lowerextremity symptoms after systemic administration of tocilizumab, an anti-interleukin-6 receptor antibody. Asian Spine Journal. 2022; 16(1):99-106. [DOI:10.31616/asj.2020.0283] [PMID] [PMCID]
- Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocrine Reviews. 2020; 41(4):594–609. [DOI:10.1210/endrev/bnaa016] [PMID] [PMCID]
- Harland N, Martin D. Exploring the longitudinal stability of the CSQ24 in a back pain population. Rehabilitation Psychology. 2014; 59(1):79-84. [DOI:10.1037/a0034337] [PMID]
- Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing-a prognostic factor for outcome in patients with low back pain: A systematic review. The Spine Journal. 2014; 14(11):2639-57. [DOI:10.1016/j.spinee.2014.03.003] [PMID]
- Ogunlana MO, Odole AC, Adejumo A, Odunaiya N. Catastrophising, pain, and disability in patients with nonspecific low back pain. Hong Kong Physiotherapy Journal. 2015; 33(2):73-9. [DOI:10.1016/j.hkpj.2015] [PMID] [PMCID]
Article type: Original Research Articles |
Subject:
Physiotherapy
Received: 2023/03/19 | Accepted: 2023/08/19 | Published: 2023/12/1
Received: 2023/03/19 | Accepted: 2023/08/19 | Published: 2023/12/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |