Volume 22, Issue 4 (December 2024)
Iranian Rehabilitation Journal 2024, 22(4): 717-728 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghazi S, Haresabadi F, Maleki Shahmahmood T. The Effect of Metabolic Disease on Language and Swallowing Skills in Children: A Case Report. Iranian Rehabilitation Journal 2024; 22 (4) :717-728
URL: http://irj.uswr.ac.ir/article-1-1960-en.html
URL: http://irj.uswr.ac.ir/article-1-1960-en.html
1- Department of Speech Therapy, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Department of Speech Pathology, School of Paramedical Sciences, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Speech Pathology, School of Paramedical Sciences, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 613 kb]
(977 Downloads)
| Abstract (HTML) (5038 Views)
Full-Text: (742 Views)
Introduction
Hyperammonemia is a metabolic disorder characterized by elevated ammonia levels in the bloodstream. It is a dangerous condition predominantly affecting the pediatric population that may lead to brain injury and death. It may be primary, caused by a genetic defect, or secondary, from various congenital and acquired factors, such as metabolic disorders [1-7]. Ammonia (NH3), the primary nitrogen source, undergoes hepatic metabolism to form urea, a nitrogenous compound excreted in urine [4, 8, 9]. Consequently, abnormally high ammonia levels in the blood lead to excessive urea production, detectable through blood tests [10]. Normal levels of ammonia in the blood vary with age [11]. Plasma ammonia levels exceed 80 µmol/L in infants up to one month old and 55 µmol/L in older children. Affected newborns typically exhibit no symptoms at birth, with the age of onset ranging from hours to months after birth. Earlier clinical onset is associated with more profound enzymatic defects [12]. Age, metabolic, nutritional status, and infections affect clinical manifestations [12, 13]. Early diagnosis and prompt treatment are essential to prevent life-threatening complications like cerebral edema and cerebral hernia. Diagnostic approaches depend on the presenting symptoms [11]. The estimated incidence of this disorder in the United States is 1 in 25000 live births [14]. However, for both children and adolescents, the global prevalence data has remained obscure [15]. Individuals with metabolic syndrome and hyperammonemia are at higher risk for other conditions, including cardiovascular issues, nutritional problems, and cognitive disorders [16, 17]. Neurological problems in hyperammonemia arise due to compromised liver function, which impairs ammonia detoxification and allows toxic substances to affect brain function.
Despite notable progress in understanding and identifying metabolic diseases such as hyperammonemia, limited knowledge exists regarding this population’s cognitive, communicative, and oral-motor functions. A literature review reveals that most studies in this field have primarily focused on the relationship between hyperammonemia and physical symptoms of metabolic disorders in children and adolescents [5, 15, 18]. Some studies have also explored cognitive impairments in affected individuals [17, 19, 20]. Patients with hyperammonemia demonstrate difficulties in memory tasks, primarily attributed to attention and visual perception deficits [21, 22]. They also exhibit poorer performance than healthy individuals in motor functions, attention, visual perception, visual orientation, and visuoconstructive abilities [23-26]. These children seem more at risk for developmental delays and problems with receptive and expressive language [27-29].
This study presents a 2.5-year-old girl diagnosed with hyperammonemia. We investigated her receptive and expressive language problems and oral-motor and swallowing dysfunctions. Also, we hope to draw the attention of specialists and physicians dealing with individuals affected by hyperammonemia to the language, cognitive, and swallowing problems these children may experience, making early speech therapy services necessary.
Case Presentation
Patient description
A previously healthy 2.5-year-old girl was brought to a rehabilitation clinic in Mahmoudabad City, Iran, for her swallowing and communication problems that started one year ago due to a metabolic disorder. The diagnosis of metabolic disorder was made at 18 months based on clinical evidence, including the results of a blood test and magnetic resonance imaging (MRI) prescribed by a neurologist.
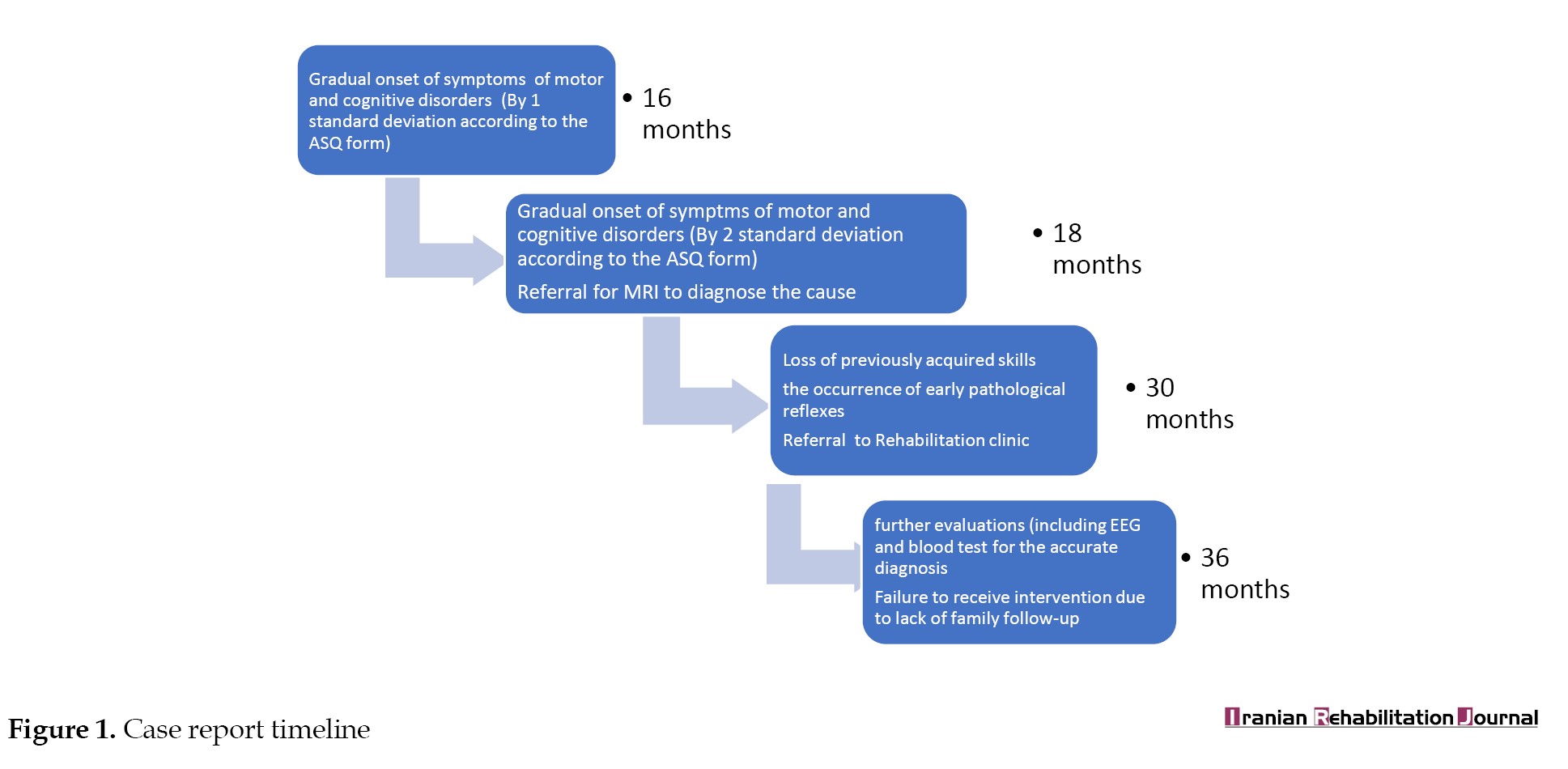
She was born to non-consanguine Iranian parents. According to parents’ reports and the ages and stages questionnaires (ASQ), she demonstrated appropriate development for her age in various domains, including gross and fine motor skills, social and communicative abilities, as well as feeding and weight gain issues up to 14 months and family history was non-contributory (Figure 1). Following the sudden onset of symptoms at 16 months old, the child was admitted to a hospital in Mahmudabad. She was brought to the emergency care department exhibiting consistent seizure activity and an altered mental state, as indicated by a Glasgow coma scale (GCS) score of 8. She was admitted to the hospital for one week, during which her seizures were managed using intravenous lorazepam. However, following her discharge, there was a gradual decline in her previously acquired motor skills, including sitting, crawling, standing, and oral motor skills related to feeding. Additionally, she experienced a loss of babbling sounds, as well as communicative abilities, which may be attributed to central nervous system (CNS) anomalies resulting from an undiagnosed metabolic deficiency. No spontaneous recovery of these skills was observed until she visited a speech therapy clinic one year later.
Clinical findings of the speech, language, and swallowing evaluations at 30 months of age demonstrated no sign of any vocalization other than sounds of crying and coughing. Pathological reflexes, including rooting and bite reflexes, were evident. The child was referred to a neurology center in Tehran City, Iran, for further evaluation to investigate the cause of the disorder. Electroencephalography (EEG) and MRI were performed and confirmed the existence of a metabolic disorder. Blood tests, endocrinologic evaluation, and genetic tests were performed at 36 months of age, and the cause was identified as hyperammonemia.
Clinical examinations
Assessment of language and communication
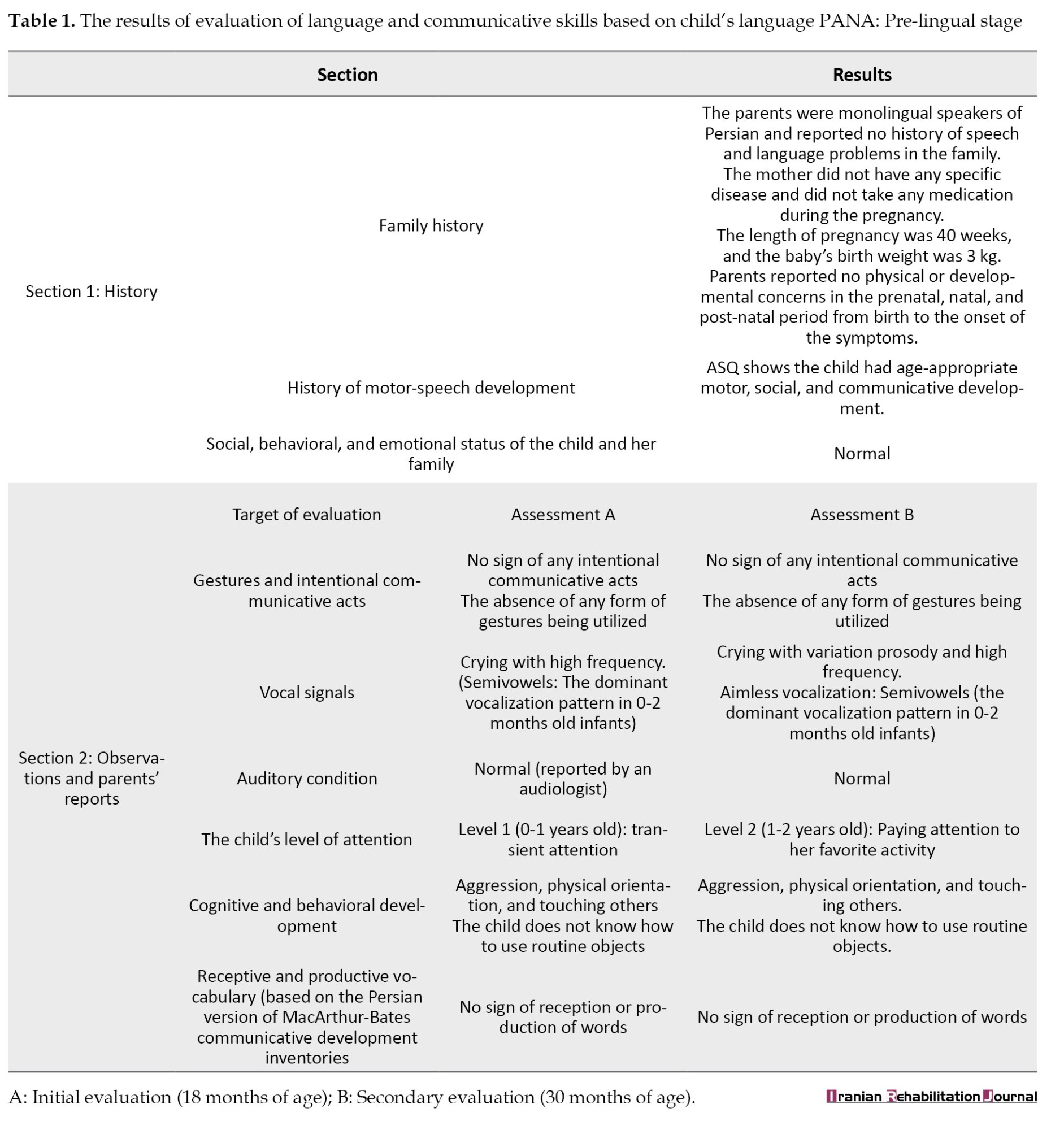
The children’s language profile monitoring package (PANA) was used to assess the child’s language and communicative skills. Because the child did not communicate verbally, a checklist for the pre-lingual stage (9-18 months) of PANA was completed. This checklist is completed based on the therapist’s observations and the parents’ reports. It contains subsections, including history (a detailed history of the child’s pre- and post-natal development and family history), the child’s plays (at 8 stages of symbolic play development) and gestures (at 3 levels of deictic, symbolic, and theatrical/co-speech gestures) assessed during the child’s free play with the parents, the child’s attention level (based on Reynell stage of attention development), and parent’s reports of the child’s feeding behaviors, vocalizations, intentional communicative acts and, receptive and expressive vocabularies (based on the Persian version of MacArthur-Bates communicative development inventories) [30-32]. Table 1 presents the results of evaluations at two different times: Initial assessment (A) at 18 months of age and the second evaluation (B) one year later.
A single examiner conducted both evaluations. The scoring and interpretation of the child’s performances were done simultaneously during the evaluation and then confirmed based on the recorded videos. Additionally, the accuracy of the scoring and interpretation of the results was confirmed by another speech therapist with at least 10 years of experience working with children with language disorders.
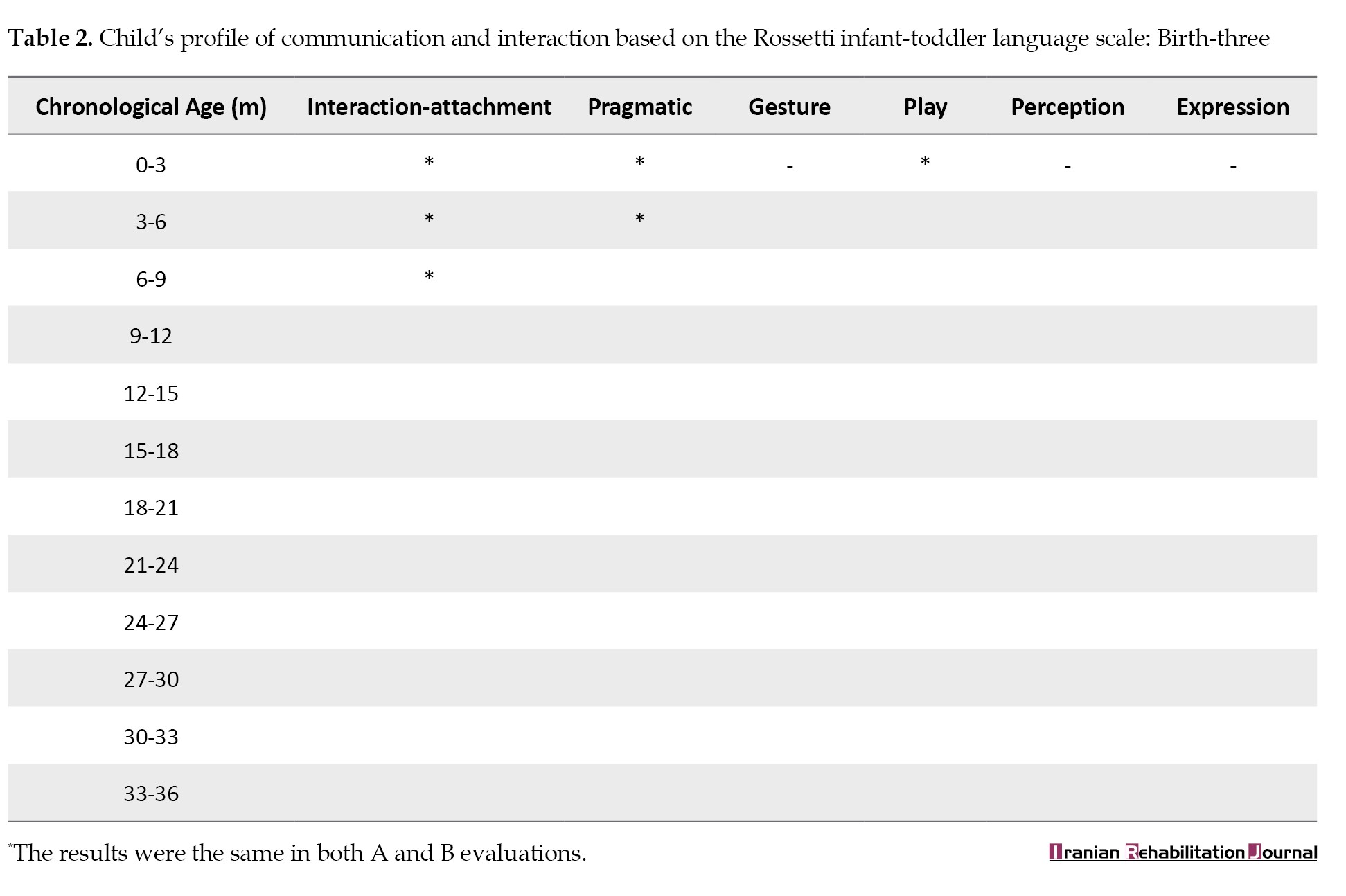
Finally, the interactive and communicative skills of the child were examined with the Persian version of Rossetti’s infant-toddler language scale, birth to three years [33]. This valid and reliable criterion-reference test measures 6 developmental domains from birth to 36 months: Interaction-attachment, pragmatics, gestures, play, language comprehension, and language expression (Table 2).
Evaluation of oral motor and feeding skills
The facial and oral cavity muscles’ structure and function were evaluated using the schedule for oral motor assessment [34], along with informal assessments performed at rest and during feeding. The results are presented in Tables 3 and 4.
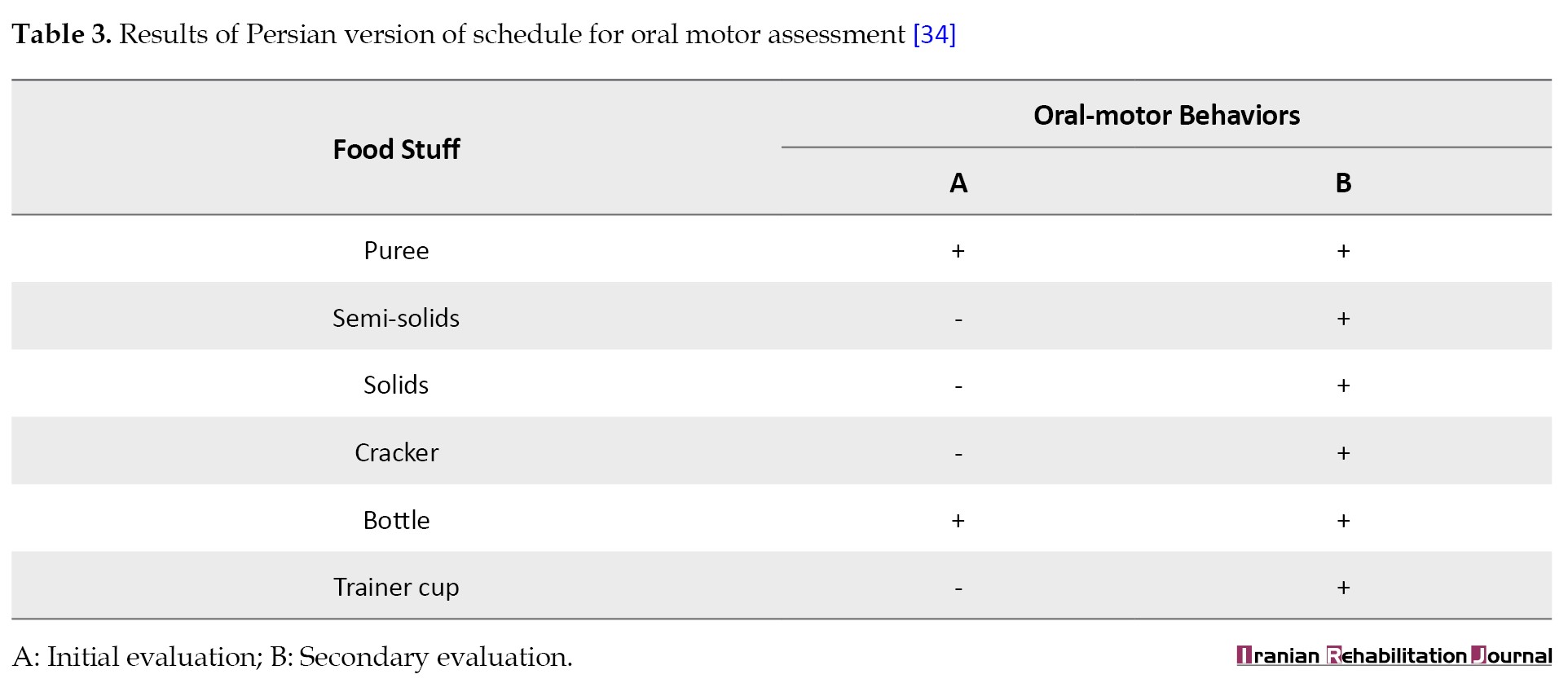
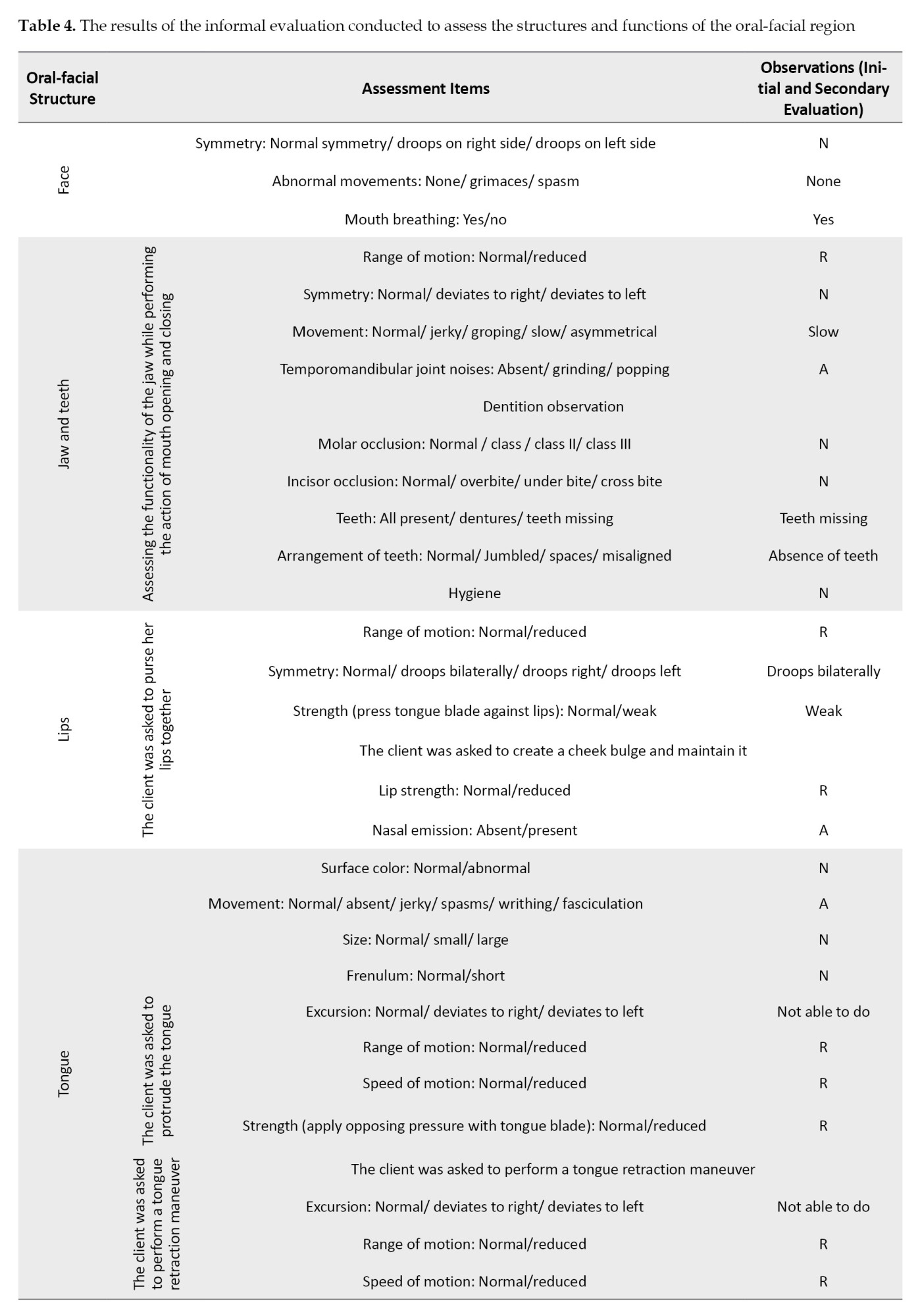
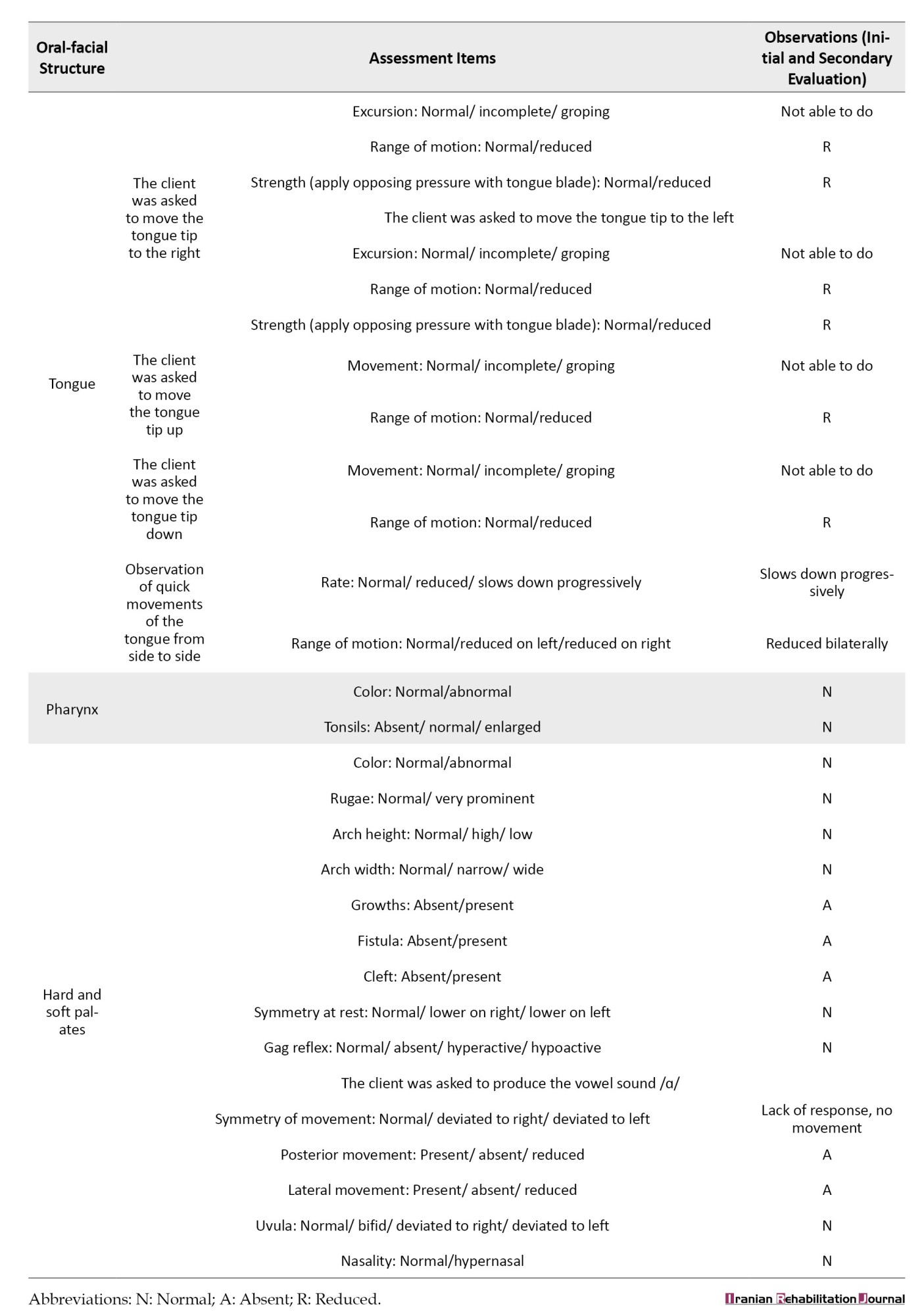
The most striking test results were related to the extreme movements of the jaw that caused open mouth posture and drooling, as well as the hypotension of the tongue muscles, which led to the child’s feeding problems.
During one year between two evaluations, the child received no interventions, such as medication, occupational therapy, or speech therapy. This condition was due to the family’s lack of follow-up.
Conclusion
While hyperammonemia is a well-known metabolic disease from the medical point of view, speech, language, cognitive, and feeding problems that affected children may experience, as well as the consequences of these disabilities on their lives and the health and social support that these children and their families require have been largely overlooked in studies. In this article, we presented a case of late-onset hyperammonemia who experienced a sudden onset of symptoms and a gradual and severe decline in cognitive, communicative, and motor functions. We aimed to highlight this disorder’s clinical manifestations and consequences for speech and language pathologists. Genetic and acquired disorders can increase ammonia levels in the blood. Urea cycle disorders are the most common cause of severe ammonia elevations, leading to recurrent, progressive, or chronic neurological disorders due to the toxic effects of ammonia accumulation and subsequent neuronal death [18]. Recurrent hyperammonemia episodes can cause a variety of symptoms, including vomiting, lethargy, coma, and even death in severe cases. Survivors may experience varying degrees of developmental disabilities, which are often correlated with the number, severity, and duration of hyperammonemia episodes, as well as the stage of brain maturation.
It has been suggested that metabolic disorders in infants, particularly disorders of the urea cycle, maybe the cause of 20% of sudden infant death syndrome cases. However, the disease may remain undiagnosed in many cases, and the infant may pass away without a definite diagnosis. It is argued that some children who display various signs of autism spectrum or behavioral disorders (such as hyperactivity accompanied by screaming, self-injury, and delirium) may be among the undiagnosed cases of urea cycle disorders [35].
Several studies have reported cases of acute hyperammonemia in individuals with late-onset inborn errors of metabolism, including previously healthy children, adolescents, and adults [36-38]. Early diagnosis and aggressive management to reduce ammonia levels in the bloodstream are essential to prevent serious complications, such as increased glutamate concentrations in the CNS, cerebral edema, increased intracranial pressure, irreversible brain damage, and psychomotor retardation [9, 35, 39, 40]. These complications can lead to various communication impairments, especially in younger children.
Motor and cognitive problems are common clinical symptoms of hyperammonemia, regardless of the underlying cause. For example, Petel et al. found these symptoms in patients with Reye syndrome [20]. Monfort et al. also showed that hyperammonemia is a major contributor to neurological alterations in patients with minimal or clinical hepatic encephalopathy, who have varying degrees of cognitive impairment [17]. A study in rats by Hernandez-Rabaza et al. reveals that hyperammonemia triggers neuroinflammation and increases GABAergic tone in the cerebellum, contributing to cognitive and motor impairment in hepatic encephalopathy [19].
Consistent with the studies that have shown that different types of metabolic disorders, including hyperammonemia, can affect cognitive and motor systems, the case presented in this study experienced acute symptoms, including seizures followed by gradual loss of motor speech as well as other communicative skills. Also, due to the weakness of the oral muscles, she was not able to suck and move his tongue and had difficulty in feeding and swallowing (Table 3). It must be noted that hyperammonemia should be considered in the differential diagnosis of encephalopathy and seizures, particularly when MRI results show symmetrical damage to the insular and cingulate cortices on both sides of the brain [40].
Some studies have documented the occurrence of motor impairments such as dystonia, ataxia, tremor, spasticity, orofacial dyskinesia, hyperkinesia, and myopathies as a result of metabolic disorders [41-43], as in our study, this child also showed signs of motor impairments such as myopathy and tremors.
Although motor disorders in children with metabolic disorders have been well-documented in the literature, there is a lack of studies documenting communication impairments in this population.
Despite the previous age-appropriate growth (based on the child’s health records and ASQ results), all social, pragmatic, and language skills in the case presented in this study were severely impaired at the assessment times. She did not play in the real sense and only looked at objects temporarily (Tables 1 and 2). This clinical profile of performances indicates severe impairments in intellectual and cognitive abilities and damage to adaptive and communicative skills resulting from likely irreversible damage to the central nervous system. Although there were slight improvements in the child’s oral-motor and language skills over time, even without direct interventions, the progress was insignificant. It could be attributed to natural maturation and or advice provided to the mother by the rehabilitation team.
Tiwari et al. retrospectively reviewed 392 case records of children with different inborn metabolic disorders. They reported that some children had communication impairments, such as delayed development of speech and language, language impairment associated with autism, hearing loss, or mental retardation, and speech and language problems associated with attention-deficit/hyperactivity disorder. Other studies have also reported communication disorders such as autism [35] and mental retardation [42, 44-46] as the general clinical presentation of metabolic disorders in children. However, there is currently a lack of research on the range of communication impairments in children with early or late-onset hyperammonemia. The oral-motor and communicative impairment observed in our case may be linked to seizures and nerve damage caused by hyperammonemia rather than being directly caused by the disorder. More studies are needed to determine the full range of communication impairments in children with hyperammonemia.
Despite the increasing awareness of metabolic disorders globally, diagnostic laboratory tests to identify some life-threatening metabolic disorders have not yet been added to the list of disorders that are screened for in newborns in Iran. Due to the limited time between the onset of initial symptoms and the onset of irreversible brain damage, rapid and effective diagnosis and treatment are critical. In the absence of mandatory screening tests that can increase the likelihood of receiving timely diagnosis and necessary services, paying particular attention to clinical symptoms is paramount for the early identification of at-risk children. General developmental delay or a decline in acquired developmental skills, including feeding and communication skills, can be the first sign in some affected children. So, attention to these signs can be crucial in the early identification of affected children, in providing adequate medical care and attention, and in reducing the extent of potential damage. Furthermore, considering the potential motor, cognitive, and communicative difficulties of children presenting with hyperammonemia, referral for a comprehensive assessment of speech, language, and communicative abilities can be a crucial step in reducing the impact of the disease on the quality of life of affected children and their families. Yet, it is possible that some parents may be unaware of the importance of screening for communication-related impairments, or physicians can sometimes miss communication disorders during routine examinations. Conducting further studies aimed at identifying the types of communication problems that individuals presenting with early- or late-onset hyperammonemia may experience and the impact that these problems have on their lives can help increase awareness of professionals who work with these children and their families, and providing appropriate and necessary services.
Conclusion
Metabolic disease caused by hyperammonemia is a rare and devastating disorder that affects all aspects of life for both the child and their family caregivers. Children and adolescents with hyperammonemia are at higher risk for other diseases, including nutritional problems and cognitive and communicative impairments. Therefore, it is crucial to pay attention to the rehabilitative evaluation and treatments following the onset of the disorder, or even slight declines in the cognitive and communicative skills that could be the first signs of a metabolic disorder.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
Thank the parents of the child presented in this study, who allowed the authors to investigate and evaluate her in-depth and share the results of these evaluations.
References
Hyperammonemia is a metabolic disorder characterized by elevated ammonia levels in the bloodstream. It is a dangerous condition predominantly affecting the pediatric population that may lead to brain injury and death. It may be primary, caused by a genetic defect, or secondary, from various congenital and acquired factors, such as metabolic disorders [1-7]. Ammonia (NH3), the primary nitrogen source, undergoes hepatic metabolism to form urea, a nitrogenous compound excreted in urine [4, 8, 9]. Consequently, abnormally high ammonia levels in the blood lead to excessive urea production, detectable through blood tests [10]. Normal levels of ammonia in the blood vary with age [11]. Plasma ammonia levels exceed 80 µmol/L in infants up to one month old and 55 µmol/L in older children. Affected newborns typically exhibit no symptoms at birth, with the age of onset ranging from hours to months after birth. Earlier clinical onset is associated with more profound enzymatic defects [12]. Age, metabolic, nutritional status, and infections affect clinical manifestations [12, 13]. Early diagnosis and prompt treatment are essential to prevent life-threatening complications like cerebral edema and cerebral hernia. Diagnostic approaches depend on the presenting symptoms [11]. The estimated incidence of this disorder in the United States is 1 in 25000 live births [14]. However, for both children and adolescents, the global prevalence data has remained obscure [15]. Individuals with metabolic syndrome and hyperammonemia are at higher risk for other conditions, including cardiovascular issues, nutritional problems, and cognitive disorders [16, 17]. Neurological problems in hyperammonemia arise due to compromised liver function, which impairs ammonia detoxification and allows toxic substances to affect brain function.
Despite notable progress in understanding and identifying metabolic diseases such as hyperammonemia, limited knowledge exists regarding this population’s cognitive, communicative, and oral-motor functions. A literature review reveals that most studies in this field have primarily focused on the relationship between hyperammonemia and physical symptoms of metabolic disorders in children and adolescents [5, 15, 18]. Some studies have also explored cognitive impairments in affected individuals [17, 19, 20]. Patients with hyperammonemia demonstrate difficulties in memory tasks, primarily attributed to attention and visual perception deficits [21, 22]. They also exhibit poorer performance than healthy individuals in motor functions, attention, visual perception, visual orientation, and visuoconstructive abilities [23-26]. These children seem more at risk for developmental delays and problems with receptive and expressive language [27-29].
This study presents a 2.5-year-old girl diagnosed with hyperammonemia. We investigated her receptive and expressive language problems and oral-motor and swallowing dysfunctions. Also, we hope to draw the attention of specialists and physicians dealing with individuals affected by hyperammonemia to the language, cognitive, and swallowing problems these children may experience, making early speech therapy services necessary.
Case Presentation
Patient description
A previously healthy 2.5-year-old girl was brought to a rehabilitation clinic in Mahmoudabad City, Iran, for her swallowing and communication problems that started one year ago due to a metabolic disorder. The diagnosis of metabolic disorder was made at 18 months based on clinical evidence, including the results of a blood test and magnetic resonance imaging (MRI) prescribed by a neurologist.
She was born to non-consanguine Iranian parents. According to parents’ reports and the ages and stages questionnaires (ASQ), she demonstrated appropriate development for her age in various domains, including gross and fine motor skills, social and communicative abilities, as well as feeding and weight gain issues up to 14 months and family history was non-contributory (Figure 1). Following the sudden onset of symptoms at 16 months old, the child was admitted to a hospital in Mahmudabad. She was brought to the emergency care department exhibiting consistent seizure activity and an altered mental state, as indicated by a Glasgow coma scale (GCS) score of 8. She was admitted to the hospital for one week, during which her seizures were managed using intravenous lorazepam. However, following her discharge, there was a gradual decline in her previously acquired motor skills, including sitting, crawling, standing, and oral motor skills related to feeding. Additionally, she experienced a loss of babbling sounds, as well as communicative abilities, which may be attributed to central nervous system (CNS) anomalies resulting from an undiagnosed metabolic deficiency. No spontaneous recovery of these skills was observed until she visited a speech therapy clinic one year later.
Clinical findings of the speech, language, and swallowing evaluations at 30 months of age demonstrated no sign of any vocalization other than sounds of crying and coughing. Pathological reflexes, including rooting and bite reflexes, were evident. The child was referred to a neurology center in Tehran City, Iran, for further evaluation to investigate the cause of the disorder. Electroencephalography (EEG) and MRI were performed and confirmed the existence of a metabolic disorder. Blood tests, endocrinologic evaluation, and genetic tests were performed at 36 months of age, and the cause was identified as hyperammonemia.
Clinical examinations
Assessment of language and communication
The children’s language profile monitoring package (PANA) was used to assess the child’s language and communicative skills. Because the child did not communicate verbally, a checklist for the pre-lingual stage (9-18 months) of PANA was completed. This checklist is completed based on the therapist’s observations and the parents’ reports. It contains subsections, including history (a detailed history of the child’s pre- and post-natal development and family history), the child’s plays (at 8 stages of symbolic play development) and gestures (at 3 levels of deictic, symbolic, and theatrical/co-speech gestures) assessed during the child’s free play with the parents, the child’s attention level (based on Reynell stage of attention development), and parent’s reports of the child’s feeding behaviors, vocalizations, intentional communicative acts and, receptive and expressive vocabularies (based on the Persian version of MacArthur-Bates communicative development inventories) [30-32]. Table 1 presents the results of evaluations at two different times: Initial assessment (A) at 18 months of age and the second evaluation (B) one year later.

A single examiner conducted both evaluations. The scoring and interpretation of the child’s performances were done simultaneously during the evaluation and then confirmed based on the recorded videos. Additionally, the accuracy of the scoring and interpretation of the results was confirmed by another speech therapist with at least 10 years of experience working with children with language disorders.
Finally, the interactive and communicative skills of the child were examined with the Persian version of Rossetti’s infant-toddler language scale, birth to three years [33]. This valid and reliable criterion-reference test measures 6 developmental domains from birth to 36 months: Interaction-attachment, pragmatics, gestures, play, language comprehension, and language expression (Table 2).

Evaluation of oral motor and feeding skills
The facial and oral cavity muscles’ structure and function were evaluated using the schedule for oral motor assessment [34], along with informal assessments performed at rest and during feeding. The results are presented in Tables 3 and 4.



The most striking test results were related to the extreme movements of the jaw that caused open mouth posture and drooling, as well as the hypotension of the tongue muscles, which led to the child’s feeding problems.
During one year between two evaluations, the child received no interventions, such as medication, occupational therapy, or speech therapy. This condition was due to the family’s lack of follow-up.
Conclusion
While hyperammonemia is a well-known metabolic disease from the medical point of view, speech, language, cognitive, and feeding problems that affected children may experience, as well as the consequences of these disabilities on their lives and the health and social support that these children and their families require have been largely overlooked in studies. In this article, we presented a case of late-onset hyperammonemia who experienced a sudden onset of symptoms and a gradual and severe decline in cognitive, communicative, and motor functions. We aimed to highlight this disorder’s clinical manifestations and consequences for speech and language pathologists. Genetic and acquired disorders can increase ammonia levels in the blood. Urea cycle disorders are the most common cause of severe ammonia elevations, leading to recurrent, progressive, or chronic neurological disorders due to the toxic effects of ammonia accumulation and subsequent neuronal death [18]. Recurrent hyperammonemia episodes can cause a variety of symptoms, including vomiting, lethargy, coma, and even death in severe cases. Survivors may experience varying degrees of developmental disabilities, which are often correlated with the number, severity, and duration of hyperammonemia episodes, as well as the stage of brain maturation.
It has been suggested that metabolic disorders in infants, particularly disorders of the urea cycle, maybe the cause of 20% of sudden infant death syndrome cases. However, the disease may remain undiagnosed in many cases, and the infant may pass away without a definite diagnosis. It is argued that some children who display various signs of autism spectrum or behavioral disorders (such as hyperactivity accompanied by screaming, self-injury, and delirium) may be among the undiagnosed cases of urea cycle disorders [35].
Several studies have reported cases of acute hyperammonemia in individuals with late-onset inborn errors of metabolism, including previously healthy children, adolescents, and adults [36-38]. Early diagnosis and aggressive management to reduce ammonia levels in the bloodstream are essential to prevent serious complications, such as increased glutamate concentrations in the CNS, cerebral edema, increased intracranial pressure, irreversible brain damage, and psychomotor retardation [9, 35, 39, 40]. These complications can lead to various communication impairments, especially in younger children.
Motor and cognitive problems are common clinical symptoms of hyperammonemia, regardless of the underlying cause. For example, Petel et al. found these symptoms in patients with Reye syndrome [20]. Monfort et al. also showed that hyperammonemia is a major contributor to neurological alterations in patients with minimal or clinical hepatic encephalopathy, who have varying degrees of cognitive impairment [17]. A study in rats by Hernandez-Rabaza et al. reveals that hyperammonemia triggers neuroinflammation and increases GABAergic tone in the cerebellum, contributing to cognitive and motor impairment in hepatic encephalopathy [19].
Consistent with the studies that have shown that different types of metabolic disorders, including hyperammonemia, can affect cognitive and motor systems, the case presented in this study experienced acute symptoms, including seizures followed by gradual loss of motor speech as well as other communicative skills. Also, due to the weakness of the oral muscles, she was not able to suck and move his tongue and had difficulty in feeding and swallowing (Table 3). It must be noted that hyperammonemia should be considered in the differential diagnosis of encephalopathy and seizures, particularly when MRI results show symmetrical damage to the insular and cingulate cortices on both sides of the brain [40].
Some studies have documented the occurrence of motor impairments such as dystonia, ataxia, tremor, spasticity, orofacial dyskinesia, hyperkinesia, and myopathies as a result of metabolic disorders [41-43], as in our study, this child also showed signs of motor impairments such as myopathy and tremors.
Although motor disorders in children with metabolic disorders have been well-documented in the literature, there is a lack of studies documenting communication impairments in this population.
Despite the previous age-appropriate growth (based on the child’s health records and ASQ results), all social, pragmatic, and language skills in the case presented in this study were severely impaired at the assessment times. She did not play in the real sense and only looked at objects temporarily (Tables 1 and 2). This clinical profile of performances indicates severe impairments in intellectual and cognitive abilities and damage to adaptive and communicative skills resulting from likely irreversible damage to the central nervous system. Although there were slight improvements in the child’s oral-motor and language skills over time, even without direct interventions, the progress was insignificant. It could be attributed to natural maturation and or advice provided to the mother by the rehabilitation team.
Tiwari et al. retrospectively reviewed 392 case records of children with different inborn metabolic disorders. They reported that some children had communication impairments, such as delayed development of speech and language, language impairment associated with autism, hearing loss, or mental retardation, and speech and language problems associated with attention-deficit/hyperactivity disorder. Other studies have also reported communication disorders such as autism [35] and mental retardation [42, 44-46] as the general clinical presentation of metabolic disorders in children. However, there is currently a lack of research on the range of communication impairments in children with early or late-onset hyperammonemia. The oral-motor and communicative impairment observed in our case may be linked to seizures and nerve damage caused by hyperammonemia rather than being directly caused by the disorder. More studies are needed to determine the full range of communication impairments in children with hyperammonemia.
Despite the increasing awareness of metabolic disorders globally, diagnostic laboratory tests to identify some life-threatening metabolic disorders have not yet been added to the list of disorders that are screened for in newborns in Iran. Due to the limited time between the onset of initial symptoms and the onset of irreversible brain damage, rapid and effective diagnosis and treatment are critical. In the absence of mandatory screening tests that can increase the likelihood of receiving timely diagnosis and necessary services, paying particular attention to clinical symptoms is paramount for the early identification of at-risk children. General developmental delay or a decline in acquired developmental skills, including feeding and communication skills, can be the first sign in some affected children. So, attention to these signs can be crucial in the early identification of affected children, in providing adequate medical care and attention, and in reducing the extent of potential damage. Furthermore, considering the potential motor, cognitive, and communicative difficulties of children presenting with hyperammonemia, referral for a comprehensive assessment of speech, language, and communicative abilities can be a crucial step in reducing the impact of the disease on the quality of life of affected children and their families. Yet, it is possible that some parents may be unaware of the importance of screening for communication-related impairments, or physicians can sometimes miss communication disorders during routine examinations. Conducting further studies aimed at identifying the types of communication problems that individuals presenting with early- or late-onset hyperammonemia may experience and the impact that these problems have on their lives can help increase awareness of professionals who work with these children and their families, and providing appropriate and necessary services.
Conclusion
Metabolic disease caused by hyperammonemia is a rare and devastating disorder that affects all aspects of life for both the child and their family caregivers. Children and adolescents with hyperammonemia are at higher risk for other diseases, including nutritional problems and cognitive and communicative impairments. Therefore, it is crucial to pay attention to the rehabilitative evaluation and treatments following the onset of the disorder, or even slight declines in the cognitive and communicative skills that could be the first signs of a metabolic disorder.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
Thank the parents of the child presented in this study, who allowed the authors to investigate and evaluate her in-depth and share the results of these evaluations.
References
- Riggio O, Merli M, Capocaccia L, Caschera M, Zullo A, Pinto G, et al. Zinc supplementation reduces blood ammonia and increases liver ornithine transcarbamylase activity in experimental cirrhosis. Hepatology (Baltimore, Md). 1992; 16(3):785-9. [DOI:10.1002/hep.1840160326 ] [PMID]
- Redant S, Beretta-Piccoli X, Mugisha A, Attou R, Kaefer K, De Bels D, et al. Hyperammonemia, the last indication of high-volume hemodiafiltration in adult and children: A structured review. Blood Purification. 2019; 48(4):330-5. [DOI:10.1159/000501390] [PMID]
- Batshaw ML, Robinson MB, Hyland K, Djali S, Heyes MP. Quinolinic acid in children with congenital hyperammonemia. Annals of Neurology. 1993; 34(5):676-81. [DOI:10.1002/ana.410340509 ] [PMID]
- Upadhyay R, Bleck TP, Busl KM. Hyperammonemia: What Urea-lly Need to Know: Case report of severe noncirrhotic hyperammonemic encephalopathy and review of the literature. Case Reports in Medicine. 2016; 2016:8512721. [DOI:10.1155/2016/8512721] [PMID]
- Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metabolic Brain Disease. 2009; 24(1):169-81. [DOI:10.1007/s11011-008-9122-5] [PMID]
- Paprocka J, Jamroz E. Hyperammonemia in children: On the crossroad of different disorders. The Neurologist. 2012; 18(5):261-5. [DOI:10.1097/NRL.0b013e318266f58a] [PMID]
- Hamed SA, Abdella MM. The risk of asymptomatic hyperammonemia in children with idiopathic epilepsy treated with valproate: Relationship to blood carnitine status. Epilepsy Research. 2009; 86(1):32-41. [DOI:10.1016/j.eplepsyres.2009.04.002 ] [PMID]
- Laub MC. [Hyperammonemia in valproate therapy in children and adolescents (German)]. Der Nervenarzt. 1986; 57(5):314-8. [PMID]
- Brusilow SW, Danney M, Waber LJ, Batshaw M, Burton B, Levitsky L, et al. Treatment of episodic hyperammonemia in children with inborn errors of urea synthesis. The New England Journal of Medicine. 1984; 310(25):1630-4. [DOI:10.1056/NEJM198406213102503] [PMID]
- Ozanne B, Nelson J, Cousineau J, Lambert M, Phan V, Mitchell G, et al. Threshold for toxicity from hyperammonemia in critically ill children. Journal of Hepatology. 2012; 56(1):123-8. [DOI:10.1016/j.jhep.2011.03.021] [PMID]
- Ali R, Nagalli S. Hyperammonemia. 2023 Apr 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. [PMID]
- Spada M, Calvo PL, Brunati A, Peruzzi L, Dell’Olio D, Romagnoli R, et al. Early liver transplantation for neonatal-onset methylmalonic acidemia. Pediatrics. 2015; 136(1):e252-6. [DOI:10.1542/peds.2015-0175 ] [PMID]
- Meyburg J, Das AM, Hoerster F, Lindner M, Kriegbaum H, Engelmann G, et al. One liver for four children: First clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation. 2009; 87(5):636-41. [DOI:10.1097/TP.0b013e318199936a ] [PMID]
- Khan A, Ayub M, Khan WM. Hyperammonemia is associated with increasing severity of both liver cirrhosis and hepatic encephalopathy. International Journal of Hepatology. 2016; 2016:6741754. [DOI:10.1155/2016/6741754 ] [PMID]
- Gebreyes YF, Goshu DY, Geletew TK, Argefa TG, Zemedu TG, Lemu KA, et al. Prevalence of high bloodpressure, hyperglycemia, dyslipidemia, metabolic syndrome and their determinants in Ethiopia: Evidences from the National NCDs STEPS Survey, 2015. PloS One. 2018; 13(5):e0194819. [DOI:10.1371/journal.pone.0194819] [PMID]
- Halfon N, Verhoef PA, Kuo AA. Childhood antecedents to adult cardiovascular disease. Pediatrics in Review. 2012; 33(2):51-60; quiz 1. [DOI:10.1542/pir.33-2-51] [PMID]
- Monfort P, Cauli O, Montoliu C, Rodrigo R, Llansola M, Piedrafita B, et al. Mechanisms of cognitive alterations in hyperammonemia and hepatic encephalopathy: therapeutical implications. Neurochemistry International. 2009; 55(1-3):106-12. [DOI:10.1016/j.neuint.2009.01.021] [PMID]
- Ribas GS, Lopes FF, Deon M, Vargas CR. Hyperammonemia in inherited metabolic diseases. Cellular and Molecular Neurobiology. 2022; 42(8):2593-610. [PMID]
- Hernandez-Rabaza V, Cabrera-Pastor A, Taoro-Gonzalez L, Gonzalez-Usano A, Agusti A, Balzano T, et al. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. Journal of Neuroinflammation. 2016; 13(1):83. [DOI:10.1186/s12974-016-0549-z] [PMID]
- Petel D, Prasad C, Rupar T, Levin S, Zizzo AN, Sharma AP, et al. Hyperammonemic encephalopathy as a manifestation of Reye syndrome in a previously-healthy 14-year-old girl: A case report. Pediatric Medicine. 2020; 3. [DOI:10.21037/pm-20-51]
- Tarter RE, Arria AM, Carra J, Van Thiel DH. Memory impairments concomitant with nonalcoholic cirrhosis. International Journal of Neuroscience. 1987; 32(3-4):853-9. [DOI:10.3109/00207458709043340] [PMID]
- Weissenborn K, Heidenreich S, Ennen J, Rückert N, Hecker H. Attention deficits in minimal hepatic encephalopathy. Metabolic Brain Disease. 2001; 16(1-2):13-9. [DOI:10.1023/A:1011654210096 ] [PMID]
- Gilberstadt SJ, Gilberstadt H, Zieve L, Buegel B, Collier RO, McClain CJ. Psychomotor performance defects in cirrhotic patients without overt encephalopathy. Archives of Internal Medicine. 1980; 140(4):519-21. [DOI:10.1001/archinte.1980.00330160079031] [PMID]
- Rehnström S, Simert G, Hansson J, Johnson G, Vang J. Chronic hepatic encephalopathy. A psychometrical study. Scandinavian Journal of Gastroenterology. 1977; 12(3):305-11. [DOI:10.3109/00365527709180932 ] [PMID]
- Rikkers L, Jenko P, Rudman D, Freides D. Subclinical hepatic encephalopathy: Detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology. 1978; 75(3):462-9. [DOI:10.1016/0016-5085(78)90851-X ] [PMID]
- Weissenborn K, Heidenreich S, Giewekemeyer K, Rückert N, Hecker H. Memory function in early hepatic encephalopathy. Journal of Hepatology. 2003; 39(3):320-5. [DOI:10.1016/S0168-8278(03)00295-2 ] [PMID]
- Dodich A, Cerami C, Inguscio E, Iannaccone S, Magnani G, Marcone A, et al. The clinico-metabolic correlates of language impairment in corticobasal syndrome and progressive supranuclear palsy. NeuroImage: Clinical. 2019; 24:102009. [DOI:10.1016/j.nicl.2019.102009 ] [PMID]
- Hassiotou F, Geddes DT. Programming of appetite control during breastfeeding as a preventative strategy against the obesity epidemic. Journal of Human Lactation: Official Journal of International Lactation Consultant Association. 2014; 30(2):136-42. [DOI:10.1177/0890334414526950] [PMID]
- Wittcopp C, Conroy R. Metabolic syndrome in children and adolescents. Pediatrics in Review. 2016; 37(5):193-202. [DOI:10.1542/pir.2014-0095 ]
- Hegde MN, Maul CA. Language disorders in children: An evidence-based approach to assessment and treatment. London: Pearson; 2006. [Link]
- Paul R. Language disorders from infancy through adolescence: Assessment & intervention. Missouri: Mosby; 2007. [Link]
- Westby C. Relationships between pretend play in preschool and later language skills. Word of Mouth. 2017; 29(1):9-11. [DOI:10.1177/1048395017726551c]
- Oryadi Zanjani M, Vahab M, Rasouli J, Ghasemi S, Yazdizade A. Center-based care and language development: A pilot study on 6-15 month-old Persian-speaking children. Journal of Rehabilitation Sciences & Research. 2016; 3(1):1-4. [DOI:10.30476/jrsr.2016.41084]
- Zarei Mahmood Abadi M, Yadegari F, Mehdizade M, Bakhshi E. Test-Retest and Inter-Rater reliability study of the schedule for oral-motor assessment in Persian Children. Iranian Rehabilitation Journal. 2018; 16(1):45-54. [DOI:10.29252/nrip.irj.16.1.45]
- Tiwari S, Kallianpur D, DeSilva KA. Communication impairments in children with inborn errors of metabolism: A preliminary study. Indian Journal of Psychological Medicine. 2017; 39(2):146-51. [DOI:10.4103/0253-7176.203125 ]
- Deodato F, Boenzi S, Rizzo C, Abeni D, Caviglia S, Picca S, et al. Inborn errors of metabolism: an update on epidemiology and on neonatal-onset hyperammonemia. Acta Paediatrica (Oslo, Norway: 1992) Supplement. 2004; 93(445):18-21. [DOI:10.1111/j.1651-2227.2004.tb03050.x ] [PMID]
- Mak CM, Lee HC, Chan AY, Lam CW. Inborn errors of metabolism and expanded newborn screening: Review and update. Critical Reviews in Clinical Laboratory Sciences. 2013; 50(6):142-62. [DOI:10.3109/10408363.2013.847896 ] [PMID]
- Saudubray JM, Garcia-Cazorla À. Inborn errors of metabolism overview: Pathophysiology, manifestations, evaluation, and management. Pediatric Clinics of North America. 2018; 65(2):179-208. [DOI:10.1016/j.pcl.2017.11.002 ] [PMID]
- Limón ID, Angulo-Cruz I, Sánchez-Abdon L, Patricio-Martínez A. Disturbance of the glutamate-glutamine cycle, secondary to hepatic damage, compromises memory function. Frontiers in Neuroscience. 2021; 15:578922. [DOI:10.3389/fnins.2021.578922 ] [PMID]
- Singh S, Koiri RK, Trigun SK. Acute and chronic hyperammonemia modulate antioxidant enzymes differently in cerebral cortex and cerebellum. Neurochemical Research. 2008; 33(1):103-13. [DOI:10.1007/s11064-007-9422-x ] [PMID]
- García-Cazorla A, Wolf NI, Serrano M, Pérez-Dueñas B, Pineda M, Campistol J, et al. Inborn errors of metabolism and motor disturbances in children. Journal of Inherited Metabolic Disease. 2009; 32(5):618-29. [DOI:10.1007/s10545-009-1194-9 ] [PMID]
- Das AM, Steuerwald U, Illsinger S. Inborn errors of energy metabolism associated with myopathies. Journal of Biomedicine & Biotechnology. 2010; 2010:340849. [DOI:10.1155/2010/340849 ] [PMID]
- Eggink H, Kuiper A, Peall KJ, Contarino MF, Bosch AM, Post B, et al. Rare inborn errors of metabolism with movement disorders: A case study to evaluate the impact upon quality of life and adaptive functioning. Orphanet Journal of rare Diseases. 2014; 9:177. [DOI:10.1186/s13023-014-0177-6 ] [PMID]
- Choudhuri T, Sengupta S. Inborn error of metabolism-An Indian perspective. International Journal of Human Genetics. 2006; 6(1):89-91. [DOI:10.31901/24566330.2006/06.01.09 ]
- Kumta NB. Inborn errors of metabolism (IEM)-an Indian perspective. The Indian Journal of Pediatrics. 2005; 72(4):325-32. [DOI:10.1007/BF02724016 ] [PMID]
- Raghuveer TS, Garg U, Graf WD. Inborn errors of metabolism in infancy and early childhood: An update. American Family Physician. 2006; 73(11):1981-90. [PMID]
Article type: Case Reports |
Subject:
Rehabilitation Management
Received: 2023/04/27 | Accepted: 2023/10/11 | Published: 2024/12/20
Received: 2023/04/27 | Accepted: 2023/10/11 | Published: 2024/12/20
Send email to the article author