Volume 23, Issue 2 (June 2025)
Iranian Rehabilitation Journal 2025, 23(2): 175-182 |
Back to browse issues page
Ethics code: IR.USWR.REC.1401.059
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kianfar M, Ershadi F S, Ahmadi Bani M. A Comparison of the Effects of Conventional Knee Ankle Foot Orthosis and TR-KAFO on Spasticity Parameters and Sleep Quality. Iranian Rehabilitation Journal 2025; 23 (2) :175-182
URL: http://irj.uswr.ac.ir/article-1-2028-en.html
URL: http://irj.uswr.ac.ir/article-1-2028-en.html
1- Department of Orthotics & Prosthetics, School of Rehabilitation Sciences, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
Keywords: Cerebral palsy, Muscle spasticity, Orthotic devices, Tone-reducing orthotics, Sleep quality
Full-Text [PDF 643 kb]
(577 Downloads)
| Abstract (HTML) (3452 Views)
Full-Text: (309 Views)
Introduction
Spasticity is prevalent and impactful in children with cerebral palsy, constituting a significant physical challenge. Its emergence involves disruptions in the sensory-motor system, involuntary muscle contractions, and reduced flexibility [1], contributing to heightened difficulties in daily activities [2]. When spasticity affects both sides of the body, the condition tends to be more severe [1]. The presence and escalation of spasticity and bothersome reflexes can disrupt a child’s sleep patterns [3]. Furthermore, it may hinder the efficacy of various rehabilitation interventions, including physical and occupational therapy interventions intended for affected individuals [4].
Spasticity has an inverse relationship with motor performance [5]. Based on clinical findings, it manifests as a series of disorders, such as hypertonia, severe reflexes, weakness, and lack of muscle coordination [6]. These disorders are managed using various treatments based on the patient’s condition [7]. These treatments include medications, injections, surgery, and rehabilitation [8]. The most common tool used to estimate spasticity in clinical conditions is the modified Ashworth scale [9]. However, more recently, the Modified Tardieu scale has been employed to assess neurological diseases and treatment in children. It is more reliable than the Modified Ashworth scale due to its superiority in metrics [10].
As a result of its long history, Orthotic therapy is one of the most widely used rehabilitation treatments for children with cerebral palsy [11]. Two types of orthoses are used in these children: Functional ankle-foot orthoses (AFO) for walking and movement [12, 13], and positional knee-ankle-foot orthoses (KAFO) for use at night and for traction [14, 15]. There are several methods for reducing spasticity, but stretching the muscles is one of the most effective [16]. KAFO is an excellent choice due to its high ability to stretch the gastrocnemius muscle at night [17]. It is believed that KAFOs provide a greater reduction in tone in children with spastic cerebral palsy when used at night than other orthoses because they immobilize and apply a wider range of traction [18]. In addition, considering the proven effect of conventional afos on decreasing tone and spasticity of limbs, the orthosis can be an important part of the treatment process for children with cerebral palsy [16]. In addition to creating immobility and tension, orthoses may reduce muscle tone by affecting the nerves. These orthoses are known as tone-reducing devices.
Tone-reducing orthoses can reduce muscle tone by adding certain elements to the orthoses [19]. This theory suggests that there are several ways to influence the tone of the lower limbs and the occurrence of abnormal muscle reflexes. The following benefits include: 1) Redistributing pressure on the soles of the feet and removing pressure from the metatarsals heads 2) Reducing pressure on tendons, particularly Achilles tendons 3) Orthokinetic effects [20]. In accordance with the prescription and time of use, night orthoses are included in CP treatment strategies. When children are sleeping, these orthoses provide an opportunity for muscles and joints to be aligned correctly and relatively calmly. Through it, therapeutic interventions are created and improved [21].
Tone-reducing orthoses are crucial for mitigating muscle tone, achieved by incorporating specific elements into the orthoses. This concept suggests various ways to influence lower limb tone and prevent abnormal muscle reflexes. The advantages of this technique include redistributing pressure on the soles of the feet, relieving pressure from the metatarsal heads [20], minimizing tension on the tendons, especially the Achilles tendons, and leveraging orthokinetic effects [19, 20].
Night orthoses are prescribed based on specific criteria and usage duration as integral components of cerebral palsy treatment. These orthoses contribute significantly during sleep, allowing for proper alignment of muscles and joints in a relatively tranquil state. This aligns with therapeutic interventions, fostering their development and refinement [21].
Appropriate nighttime sleep is critical due to its direct impact on neurophysiological function and learning. Sleep disorders are more prevalent in children with cerebral palsy than in normal children [22, 23], which is a significant reason to aim to improve the quality of sleep in these children [8]. Understanding the correlation between sleep disturbances and muscle spasticity is crucial for assessing overall sleep quality [24].
Orthoses seem to disrupt sleep, a concern with limited dedicated studies [22]. A significant gap is found in research regarding the impact of tone-reducing KAFOs (TR-KAFOs) on the sleep quality of children with cerebral palsy, especially when used at night. No study has been conducted on applying TR-KAFOs in children with cerebral palsy. Given the multifactorial nature of sleep disturbances, where involuntary muscle activity plays a significant role [1], assessing muscle spasticity alongside sleep disturbance measurements allows for a more comprehensive understanding of the factors affecting sleep quality. Therefore, this study aimed to assess the influence of TR-KAFOs on sleep quality and spasticity in this population.
There are a variety of measures to assess sleep quality, and the general psychometric properties of the sleep disturbance scale for children (SDSC) questionnaire, including internal consistency and convergent validity, appears more appropriate for this population [25].
Materials and Methods
Participants and sample size
The inclusion criteria included 12 patients aged 3-13 years with spastic dipelgia cerebral palsy [26] with gross motor function 2 or 3 and Ashworth 1+ or 2, prescribed with a KAFO, without infection or skin inflammation, and treated with neuroleptics.
The gross motor function classification system (GMFCS) is a standard classification [27] for classifying children with cerebral palsy motor functional abilities. This classification has significant functional limitations that are used to prescribe assistive devices. Children in this category are classified as grades 1-5. Individuals with GMFCS levels 2 or 3 are candidates for orthoses [27].
To evaluate spasticity, the modified Tardieu scale was used before and after four weeks [28]. We used the SDSC questionnaire to assess sleep quality [4]. G-power calculated twelve participants [29].
According to the ratio of Hoffman’s reflex to motor feedback after knee-ankle-foot orthosis intervention on the gastrocnemius muscle, the change in the first group was 0.59 and the standard deviation was 0.12, whereas the change in the second group was 0.33 and the standard deviation was 0.13. In conclusion, the sample size calculations indicated that both groups would require 12 participants.
Intervention
In the control group, regular KAFO users wore an orthosis similar to a KAFO that reduced tone without affecting spasticity (Figure 1).

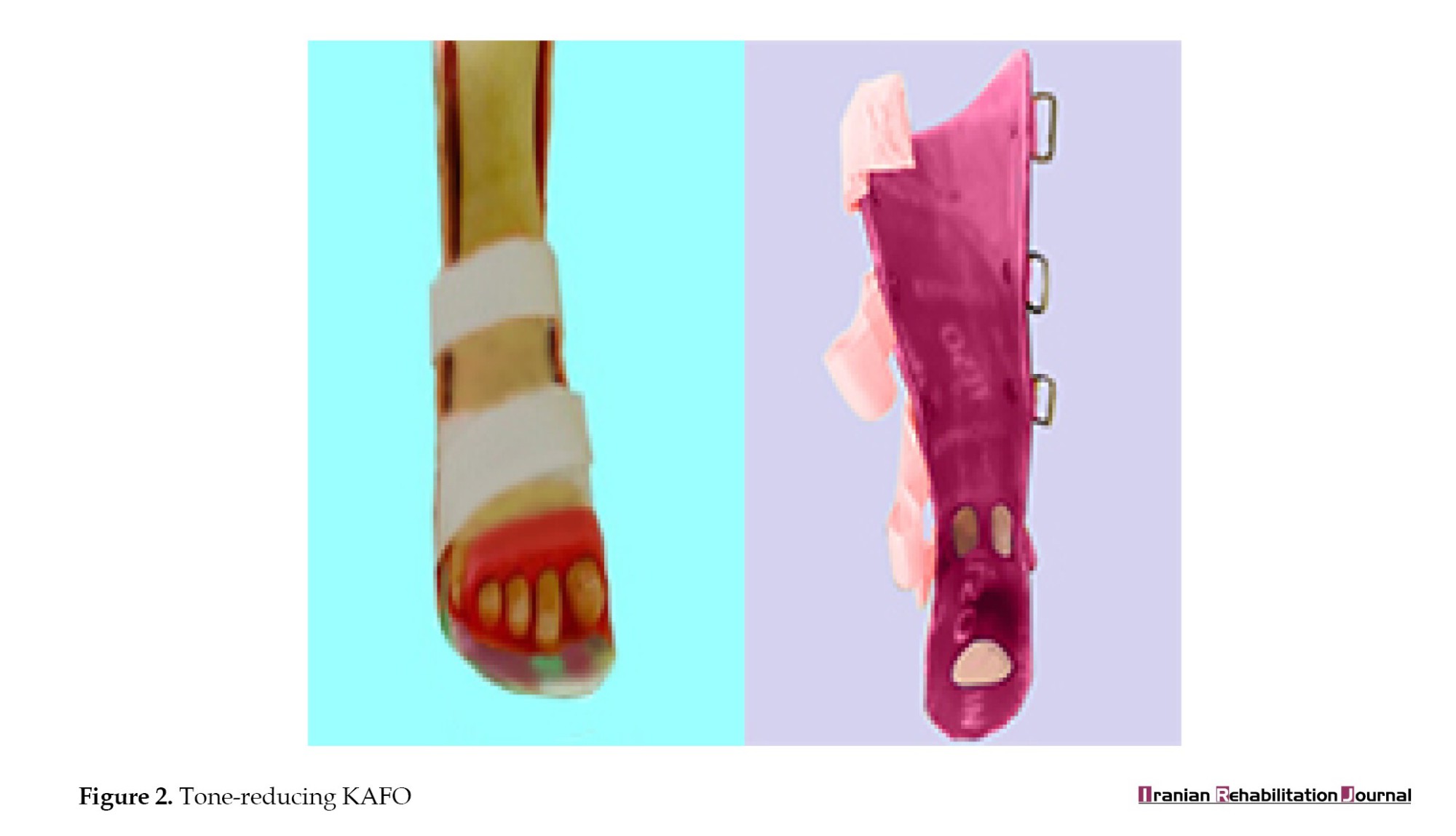
In the case group, KAFO was used along with tone-reducing attachments, such as metatarsal pads and toe separators [6, 20] to control plantar reflexes [28], heel immobilization was applied, and pressure was exerted on the Achilles tendon to activate the Golgi system [28, 29]. Orthokinetic properties were also applied to eliminate annoying plantar reflexes [20] (Figure 2).
Data collection
The primary variables were the Tardieu measurement scale at two speeds (V1 and V3) in the gastrocnemius muscle [10]. The Tardieu scale has demonstrated good reliability, meaning that it consistently produces similar results when used by different raters or in different situations. Studies, such as that by Yoo et al. [2] have shown reliable outcomes when used by experienced and inexperienced raters. In terms of validity, the Tardieu scale has been effectively identified the spasticity’s presence. Alhusaini et al. [3] demonstrated that the Tardieu Scale was more effective than the Ashworth scale in identifying the presence of spasticity in children. For evaluation, the patients were in the supine position with the knee semi-flexed, and passive stretches were performed at specified velocities to record data before and after treatment.
We also measured sleep quality using the SDSC in two groups before and after the intervention. The SDSC has undergone rigorous validation [4] and is highly reliable for measuring sleep disturbances [30]. This tool includes disorders of initiating and maintaining sleep, sleep breathing disorders, disorders of arousal, sleep-wake transition disorders, and parasomnias.
As a part of the study, we examined sleep quality data before and after the intervention. We also examined the differences between the two groups before and after the intervention. This study examined the normality of the data. In the last four weeks of the study, parents completed the summary questionnaire on sleep disorders and provided us with their responses. Following the study, data on sleep quality were collected based on the four-week study duration to allow comparisons [26]. Additionally, spasticity data were collected by an expert at two stages before and after the study period. This was done according to Tardieu’s criteria. Data were first collected and categorized using Excel software, and then statistically analysed using SPSS software, version 26.
Study protocol
We explained the procedures for conducting the test to the participants in detail, and parents and children consented. All participants were randomly allocated to two groups: Conventional and TR KAFOs. KAFOs were designed and fabricated by a certified orthotist. All data were recorded before and after the intervention. All participants were advised to use their devices for at least 6 hours a night for four weeks.
Before grouping the children and conducting the study, we measured their spasticity and sleep quality. Two conditions were examined in this study: 1) a normal night KAFO and 2) a tone-reducing night KAFO. The grouping was not known to either the control group or the intervention group. Each KAFO was designed and manufactured by an certified orthotist. To ensure that the test would not have any psychological impact on the subject, a placebo was also used in the control group. In the control group, patients were given a regular night KAFO orthosis that had no effect on tone reduction compared to a tone-reducing night KAFO orthosis. Following the intervention, patients’ spasticity and sleep quality were remeasured, and data were collected. They were required to wear the orthosis for a minimum of six hours a night [21] for four weeks [26].
Statistical analysis
SPSS software, version 26.0 was used for statistical analysis. The Shapiro-Wilk test demonstrated variable normality. Since the spasticity data were not parametric, the Mann-Whitney test was used to compare the two groups. Wilcoxon was used to evaluate the effect of orthosis before and after the study was initiated. The sleep quality was compared between the two groups using an independent t-tests and a paired t-test before and after the study. All analyses were conducted at a significance level of α=0.05.
Results
A study was conducted to estimate sleep disorders’ Mean±SD in conventional and TR-KAFOs. In this study, a decrease in the score indicated better sleep quality. The Mean±SD changed from 52.83±6.616 to 45.67±6.377, indicating that the observation score decreased after the intervention group was administered TR-KAFO after 4 weeks. This change was significant (P<0.05). A significant difference was also observed between the two groups when comparing the same period of the two orthoses (P<0.05). However, the change in sleep quality scores in the control group was not noticeable compared to the results before and after the study (P>0.05).As a result of the non-parametric nature of the data, quartiles and median were also used in the spasticity analysis. In the intervention group, the degree of spasticity reduction was significant (P<0.05). Also, a significant difference was observed between the results obtained from the two groups at the end of the study (P<0.05). However, with the investigation performed in the control group, the spasticity change was not noticeable (P>0.05). According to the study, TR-KAFO was more effective than conventional KAFO at improving sleep quality and reducing spasticity in patients.
Discussion
Although ankle orthoses have been evaluated differently for upper motor neuron patients, the findings cannot be generalized to KAFO. In this study, the effect of TR-KAFO on sleep quality and tone reduction was examined. Stretching the leg muscles is a crucial measure employed by KAFO to alleviate spasticity [18]. An effective way to alleviate spasticity symptoms is to stretch the muscles [31]. Additionally, tone-reducing features can minimize muscle tone and enhance motor control and reflexes [16]. Increased motor control and reflexes improve individual performance [19]. Achilles tendon pressure, metatarsal pads, and toe separators affect nerve stimulation and control reflexes. Another difference between the two groups was that orthokinetic properties were affected, which are determined by the type of orthotic material that comes into contact with the skin [20].
Sleep quality is paramount because it is a crucial factor in children’s development and growth [32]. Furthermore, considering the four times higher prevalence of sleep disorders in children with cerebral palsy [33], it has detrimental effects on parents’ sleep and daily activities [32]. Based on the assumption that reducing spasticity improves sleep quality, in our study, the combination of tone reduction features with KAFO reduced muscle tone and improved sleep quality in children with spastic diplegic cerebral palsy.
TR-KAFO was more effective in reducing spasticity than conventional KAFO when used overnight. The Hoffman and Ashworth reflex scale was used in another study to determine whether regular nocturnal KAFO reduces spasticity. According to this study, KAFO was more effective than AFO in reducing spasticity [32]. Tone-reducing elements were not included in this study to determine whether adding tone-reducing features to the orthosis could further decrease spasticity. As a result of its positive effects, TR-KAFO may increase the effectiveness of KAFO by decreasing spasticity. Another study examined the effect of adding the tone-reducing property of the AFO on walking parameters and found that it did not affect walking quality [26]. This conclusion may be attributed to the difference in examining the orthosis in a dynamic state, whereas in our study, we observed the orthosis in a non-dynamic state. Also, considering that reduction in spasticity with its secondary effects may also improve walking conditions, this conclusion may be a result of a difference in the study population and perhaps a difference in the type of orthoses utilized.
As compared to conventional KAFO, TR-KAFO improved sleep quality in our study. Nevertheless, another study concluded that orthoses did not affect sleep quality [21]. This conclusion was attributed to the difference in disease intensity in their study. Furthermore, a conventional orthosis without tone-reducing properties was used in this study. In our study, orthoses with more comfortable designs improved sleep. This may provide evidence better understanding of the comfort of an orthosis with the ability to reduce tone [34].
We found that TR-KAFO reduced gastrocnemius muscle spasticity and improved sleep quality. However, this study had a few limitations. First, no study has investigated the tone-reducing effect of the TR-KAFO, and only a small number of studies have investigated the tone-reducing effect of positional orthoses. Second, the time the orthosis was used at night may not be accurate, given the lack of accurate monitoring. Finally, occupational therapy was provided to the patients.
This study has several limitations. Insufficient research on TR-KAFO’s tone-reducing effect: The study is constrained by a lack of comprehensive research on the TR-KAFO’s tone-reducing impact. More extensive investigations are required in this specific area to enhance our understanding.
Short-term follow-up: The study’s short-term follow-up duration may hinder capturing long-term implications and sustainability of TR-KAFO effects. Extended follow-up periods in future studies could provide a more comprehensive picture of the intervention’s outcomes.
Lack of more accurate tools for measuring muscle tone: This study recognized the absence of precise tools for measuring muscle tone, emphasizing the need for advancements in assessment methodologies to enhance accuracy.
In summary, addressing these limitations in future research will contribute to a more nuanced and reliable comprehension of TR-KAFO’s effectiveness.
Conclusion
While orthoses alone reduce spasticity, their impact on sleep quality was unknown. In addition, there has been no study of tone-reducing elements in night orthoses, especially when children use KAFO. For further analysis of the efficacy of night orthoses in decreasing spasticity and improving sleep quality, further studies are required. Furthermore, it is important to investigate the effects of different orthoses as well as optimal night orthoses design.
In this study, we concluded that in children with spastic cerebral palsy, TR-KAFOs could decrease spasticity and improve sleep quality after a 4 weeks intervention. elements were added to the night KAFO orthosis, leading to decreased spasticity and improved sleep quality. These results suggest that the use of an orthotic device with tone-reducing elements can provide a simple and effective solution to improve sleep quality and decrease spasticity in children with cerebral palsy. Further, such devices can assist in reducing the need for more aggressive treatments in the future.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of University of Social Welfare and Rehabilitation Sciences, Tehran, Iran (Code: IR.USWR.REC.1401.059).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Hami and Nikan Occupational Therapy Clinics for their support and cooperation.
References
Spasticity is prevalent and impactful in children with cerebral palsy, constituting a significant physical challenge. Its emergence involves disruptions in the sensory-motor system, involuntary muscle contractions, and reduced flexibility [1], contributing to heightened difficulties in daily activities [2]. When spasticity affects both sides of the body, the condition tends to be more severe [1]. The presence and escalation of spasticity and bothersome reflexes can disrupt a child’s sleep patterns [3]. Furthermore, it may hinder the efficacy of various rehabilitation interventions, including physical and occupational therapy interventions intended for affected individuals [4].
Spasticity has an inverse relationship with motor performance [5]. Based on clinical findings, it manifests as a series of disorders, such as hypertonia, severe reflexes, weakness, and lack of muscle coordination [6]. These disorders are managed using various treatments based on the patient’s condition [7]. These treatments include medications, injections, surgery, and rehabilitation [8]. The most common tool used to estimate spasticity in clinical conditions is the modified Ashworth scale [9]. However, more recently, the Modified Tardieu scale has been employed to assess neurological diseases and treatment in children. It is more reliable than the Modified Ashworth scale due to its superiority in metrics [10].
As a result of its long history, Orthotic therapy is one of the most widely used rehabilitation treatments for children with cerebral palsy [11]. Two types of orthoses are used in these children: Functional ankle-foot orthoses (AFO) for walking and movement [12, 13], and positional knee-ankle-foot orthoses (KAFO) for use at night and for traction [14, 15]. There are several methods for reducing spasticity, but stretching the muscles is one of the most effective [16]. KAFO is an excellent choice due to its high ability to stretch the gastrocnemius muscle at night [17]. It is believed that KAFOs provide a greater reduction in tone in children with spastic cerebral palsy when used at night than other orthoses because they immobilize and apply a wider range of traction [18]. In addition, considering the proven effect of conventional afos on decreasing tone and spasticity of limbs, the orthosis can be an important part of the treatment process for children with cerebral palsy [16]. In addition to creating immobility and tension, orthoses may reduce muscle tone by affecting the nerves. These orthoses are known as tone-reducing devices.
Tone-reducing orthoses can reduce muscle tone by adding certain elements to the orthoses [19]. This theory suggests that there are several ways to influence the tone of the lower limbs and the occurrence of abnormal muscle reflexes. The following benefits include: 1) Redistributing pressure on the soles of the feet and removing pressure from the metatarsals heads 2) Reducing pressure on tendons, particularly Achilles tendons 3) Orthokinetic effects [20]. In accordance with the prescription and time of use, night orthoses are included in CP treatment strategies. When children are sleeping, these orthoses provide an opportunity for muscles and joints to be aligned correctly and relatively calmly. Through it, therapeutic interventions are created and improved [21].
Tone-reducing orthoses are crucial for mitigating muscle tone, achieved by incorporating specific elements into the orthoses. This concept suggests various ways to influence lower limb tone and prevent abnormal muscle reflexes. The advantages of this technique include redistributing pressure on the soles of the feet, relieving pressure from the metatarsal heads [20], minimizing tension on the tendons, especially the Achilles tendons, and leveraging orthokinetic effects [19, 20].
Night orthoses are prescribed based on specific criteria and usage duration as integral components of cerebral palsy treatment. These orthoses contribute significantly during sleep, allowing for proper alignment of muscles and joints in a relatively tranquil state. This aligns with therapeutic interventions, fostering their development and refinement [21].
Appropriate nighttime sleep is critical due to its direct impact on neurophysiological function and learning. Sleep disorders are more prevalent in children with cerebral palsy than in normal children [22, 23], which is a significant reason to aim to improve the quality of sleep in these children [8]. Understanding the correlation between sleep disturbances and muscle spasticity is crucial for assessing overall sleep quality [24].
Orthoses seem to disrupt sleep, a concern with limited dedicated studies [22]. A significant gap is found in research regarding the impact of tone-reducing KAFOs (TR-KAFOs) on the sleep quality of children with cerebral palsy, especially when used at night. No study has been conducted on applying TR-KAFOs in children with cerebral palsy. Given the multifactorial nature of sleep disturbances, where involuntary muscle activity plays a significant role [1], assessing muscle spasticity alongside sleep disturbance measurements allows for a more comprehensive understanding of the factors affecting sleep quality. Therefore, this study aimed to assess the influence of TR-KAFOs on sleep quality and spasticity in this population.
There are a variety of measures to assess sleep quality, and the general psychometric properties of the sleep disturbance scale for children (SDSC) questionnaire, including internal consistency and convergent validity, appears more appropriate for this population [25].
Materials and Methods
Participants and sample size
The inclusion criteria included 12 patients aged 3-13 years with spastic dipelgia cerebral palsy [26] with gross motor function 2 or 3 and Ashworth 1+ or 2, prescribed with a KAFO, without infection or skin inflammation, and treated with neuroleptics.
The gross motor function classification system (GMFCS) is a standard classification [27] for classifying children with cerebral palsy motor functional abilities. This classification has significant functional limitations that are used to prescribe assistive devices. Children in this category are classified as grades 1-5. Individuals with GMFCS levels 2 or 3 are candidates for orthoses [27].
To evaluate spasticity, the modified Tardieu scale was used before and after four weeks [28]. We used the SDSC questionnaire to assess sleep quality [4]. G-power calculated twelve participants [29].
According to the ratio of Hoffman’s reflex to motor feedback after knee-ankle-foot orthosis intervention on the gastrocnemius muscle, the change in the first group was 0.59 and the standard deviation was 0.12, whereas the change in the second group was 0.33 and the standard deviation was 0.13. In conclusion, the sample size calculations indicated that both groups would require 12 participants.
Intervention
In the control group, regular KAFO users wore an orthosis similar to a KAFO that reduced tone without affecting spasticity (Figure 1).

In the case group, KAFO was used along with tone-reducing attachments, such as metatarsal pads and toe separators [6, 20] to control plantar reflexes [28], heel immobilization was applied, and pressure was exerted on the Achilles tendon to activate the Golgi system [28, 29]. Orthokinetic properties were also applied to eliminate annoying plantar reflexes [20] (Figure 2).

Data collection
The primary variables were the Tardieu measurement scale at two speeds (V1 and V3) in the gastrocnemius muscle [10]. The Tardieu scale has demonstrated good reliability, meaning that it consistently produces similar results when used by different raters or in different situations. Studies, such as that by Yoo et al. [2] have shown reliable outcomes when used by experienced and inexperienced raters. In terms of validity, the Tardieu scale has been effectively identified the spasticity’s presence. Alhusaini et al. [3] demonstrated that the Tardieu Scale was more effective than the Ashworth scale in identifying the presence of spasticity in children. For evaluation, the patients were in the supine position with the knee semi-flexed, and passive stretches were performed at specified velocities to record data before and after treatment.
We also measured sleep quality using the SDSC in two groups before and after the intervention. The SDSC has undergone rigorous validation [4] and is highly reliable for measuring sleep disturbances [30]. This tool includes disorders of initiating and maintaining sleep, sleep breathing disorders, disorders of arousal, sleep-wake transition disorders, and parasomnias.
As a part of the study, we examined sleep quality data before and after the intervention. We also examined the differences between the two groups before and after the intervention. This study examined the normality of the data. In the last four weeks of the study, parents completed the summary questionnaire on sleep disorders and provided us with their responses. Following the study, data on sleep quality were collected based on the four-week study duration to allow comparisons [26]. Additionally, spasticity data were collected by an expert at two stages before and after the study period. This was done according to Tardieu’s criteria. Data were first collected and categorized using Excel software, and then statistically analysed using SPSS software, version 26.
Study protocol
We explained the procedures for conducting the test to the participants in detail, and parents and children consented. All participants were randomly allocated to two groups: Conventional and TR KAFOs. KAFOs were designed and fabricated by a certified orthotist. All data were recorded before and after the intervention. All participants were advised to use their devices for at least 6 hours a night for four weeks.
Before grouping the children and conducting the study, we measured their spasticity and sleep quality. Two conditions were examined in this study: 1) a normal night KAFO and 2) a tone-reducing night KAFO. The grouping was not known to either the control group or the intervention group. Each KAFO was designed and manufactured by an certified orthotist. To ensure that the test would not have any psychological impact on the subject, a placebo was also used in the control group. In the control group, patients were given a regular night KAFO orthosis that had no effect on tone reduction compared to a tone-reducing night KAFO orthosis. Following the intervention, patients’ spasticity and sleep quality were remeasured, and data were collected. They were required to wear the orthosis for a minimum of six hours a night [21] for four weeks [26].
Statistical analysis
SPSS software, version 26.0 was used for statistical analysis. The Shapiro-Wilk test demonstrated variable normality. Since the spasticity data were not parametric, the Mann-Whitney test was used to compare the two groups. Wilcoxon was used to evaluate the effect of orthosis before and after the study was initiated. The sleep quality was compared between the two groups using an independent t-tests and a paired t-test before and after the study. All analyses were conducted at a significance level of α=0.05.
Results
A study was conducted to estimate sleep disorders’ Mean±SD in conventional and TR-KAFOs. In this study, a decrease in the score indicated better sleep quality. The Mean±SD changed from 52.83±6.616 to 45.67±6.377, indicating that the observation score decreased after the intervention group was administered TR-KAFO after 4 weeks. This change was significant (P<0.05). A significant difference was also observed between the two groups when comparing the same period of the two orthoses (P<0.05). However, the change in sleep quality scores in the control group was not noticeable compared to the results before and after the study (P>0.05).As a result of the non-parametric nature of the data, quartiles and median were also used in the spasticity analysis. In the intervention group, the degree of spasticity reduction was significant (P<0.05). Also, a significant difference was observed between the results obtained from the two groups at the end of the study (P<0.05). However, with the investigation performed in the control group, the spasticity change was not noticeable (P>0.05). According to the study, TR-KAFO was more effective than conventional KAFO at improving sleep quality and reducing spasticity in patients.
Discussion
Although ankle orthoses have been evaluated differently for upper motor neuron patients, the findings cannot be generalized to KAFO. In this study, the effect of TR-KAFO on sleep quality and tone reduction was examined. Stretching the leg muscles is a crucial measure employed by KAFO to alleviate spasticity [18]. An effective way to alleviate spasticity symptoms is to stretch the muscles [31]. Additionally, tone-reducing features can minimize muscle tone and enhance motor control and reflexes [16]. Increased motor control and reflexes improve individual performance [19]. Achilles tendon pressure, metatarsal pads, and toe separators affect nerve stimulation and control reflexes. Another difference between the two groups was that orthokinetic properties were affected, which are determined by the type of orthotic material that comes into contact with the skin [20].
Sleep quality is paramount because it is a crucial factor in children’s development and growth [32]. Furthermore, considering the four times higher prevalence of sleep disorders in children with cerebral palsy [33], it has detrimental effects on parents’ sleep and daily activities [32]. Based on the assumption that reducing spasticity improves sleep quality, in our study, the combination of tone reduction features with KAFO reduced muscle tone and improved sleep quality in children with spastic diplegic cerebral palsy.
TR-KAFO was more effective in reducing spasticity than conventional KAFO when used overnight. The Hoffman and Ashworth reflex scale was used in another study to determine whether regular nocturnal KAFO reduces spasticity. According to this study, KAFO was more effective than AFO in reducing spasticity [32]. Tone-reducing elements were not included in this study to determine whether adding tone-reducing features to the orthosis could further decrease spasticity. As a result of its positive effects, TR-KAFO may increase the effectiveness of KAFO by decreasing spasticity. Another study examined the effect of adding the tone-reducing property of the AFO on walking parameters and found that it did not affect walking quality [26]. This conclusion may be attributed to the difference in examining the orthosis in a dynamic state, whereas in our study, we observed the orthosis in a non-dynamic state. Also, considering that reduction in spasticity with its secondary effects may also improve walking conditions, this conclusion may be a result of a difference in the study population and perhaps a difference in the type of orthoses utilized.
As compared to conventional KAFO, TR-KAFO improved sleep quality in our study. Nevertheless, another study concluded that orthoses did not affect sleep quality [21]. This conclusion was attributed to the difference in disease intensity in their study. Furthermore, a conventional orthosis without tone-reducing properties was used in this study. In our study, orthoses with more comfortable designs improved sleep. This may provide evidence better understanding of the comfort of an orthosis with the ability to reduce tone [34].
We found that TR-KAFO reduced gastrocnemius muscle spasticity and improved sleep quality. However, this study had a few limitations. First, no study has investigated the tone-reducing effect of the TR-KAFO, and only a small number of studies have investigated the tone-reducing effect of positional orthoses. Second, the time the orthosis was used at night may not be accurate, given the lack of accurate monitoring. Finally, occupational therapy was provided to the patients.
This study has several limitations. Insufficient research on TR-KAFO’s tone-reducing effect: The study is constrained by a lack of comprehensive research on the TR-KAFO’s tone-reducing impact. More extensive investigations are required in this specific area to enhance our understanding.
Short-term follow-up: The study’s short-term follow-up duration may hinder capturing long-term implications and sustainability of TR-KAFO effects. Extended follow-up periods in future studies could provide a more comprehensive picture of the intervention’s outcomes.
Lack of more accurate tools for measuring muscle tone: This study recognized the absence of precise tools for measuring muscle tone, emphasizing the need for advancements in assessment methodologies to enhance accuracy.
In summary, addressing these limitations in future research will contribute to a more nuanced and reliable comprehension of TR-KAFO’s effectiveness.
Conclusion
While orthoses alone reduce spasticity, their impact on sleep quality was unknown. In addition, there has been no study of tone-reducing elements in night orthoses, especially when children use KAFO. For further analysis of the efficacy of night orthoses in decreasing spasticity and improving sleep quality, further studies are required. Furthermore, it is important to investigate the effects of different orthoses as well as optimal night orthoses design.
In this study, we concluded that in children with spastic cerebral palsy, TR-KAFOs could decrease spasticity and improve sleep quality after a 4 weeks intervention. elements were added to the night KAFO orthosis, leading to decreased spasticity and improved sleep quality. These results suggest that the use of an orthotic device with tone-reducing elements can provide a simple and effective solution to improve sleep quality and decrease spasticity in children with cerebral palsy. Further, such devices can assist in reducing the need for more aggressive treatments in the future.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of University of Social Welfare and Rehabilitation Sciences, Tehran, Iran (Code: IR.USWR.REC.1401.059).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors thank the Hami and Nikan Occupational Therapy Clinics for their support and cooperation.
References
- Karabulut D, Avcı Ş. Relationship between sleep problems and gross motor function in children with cerebral palsy and investigation of their parents’ quality of life. Turkish Journal of Physiotherapy and Rehabilitation. 2020; 31(2):180-7. [DOI:10.21653/tjpr.535131]
- Yoo M, Ahn JH, Rha DW, Park ES. Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy. Children (Basel, Switzerland). 2022; 9(6):827. [DOI:10.3390/children9060827] [PMID]
- Alhusaini AA, Dean CM, Crosbie J, Shepherd RB, Lewis J. Evaluation of spasticity in children with cerebral palsy using Ashworth and Tardieu scales compared with laboratory measures. Journal of Child Neurology. 2010; 25(10):1242-7. [DOI:10.1177/0883073810362266] [PMID]
- Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The sleep disturbance scale for children (SDSC) construct ion and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. Journal of Sleep Research. 1996; 5(4):251-61. [DOI:10.1111/j.1365-2869.1996.00251.x] [PMID]
- Jo HM, Song JC, Jang SH. Improvements in spasticity and motor function using a static stretching device for people with chronic hemiparesis following stroke. NeuroRehabilitation. 2013; 32(2):369-75. [DOI:10.3233/NRE-130857] [PMID]
- Nash B, Roller JM, Parker MG. The effects of tone-reducing orthotics on walking of an individual after incomplete spinal cord injury. Journal of Neurologic Physical Therapy. 2008; 32(1):39-47. [DOI:10.1097/NPT.0b013e3181659556] [PMID]
- Goldstein EM. Spasticity management: An overview. Journal of Child Neurology. 2001; 16(1):16-23. [DOI:10.1177/088307380101600104] [PMID]
- Cobeljic G, Bumbasirevic M, Lesic A, Bajin Z. The management of spastic equinus in cerebral palsy. Orthopaedics and Trauma. 2009; 23(3):201-9. [DOI:10.1016/j.mporth.2009.05.003]
- Mutlu A, Livanelioglu A, Gunel MK. Reliability of goniometric measurements in children with spastic cerebral palsy. Medical Science Monitor. 2007; 13(7):329-9. [PMID]
- Morris S. Ashworth and Tardieu Scales: Their clinical relevance for measuring spasticity in adult and paediatric neurological populations. Physical Therapy Reviews. 2002; 7(1):53-62. [DOI:10.1179/108331902125001770]
- Morris C. A review of the efficacy of lower-limb orthoses used for cerebral palsy. Developmental Medicine and Child Neurology. 2002; 44(3):205-11. [PMID]
- Carlson WE, Vaughan CL, Damiano DL, Abel MF. Orthotic management of gait in spastic diplegia1. American Journal of Physical Medicine & Rehabilitation. 1997; 76(3):219-25. [DOI:10.1097/00002060-199705000-00012] [PMID]
- Chisholm AE, Perry SD. Ankle-foot orthotic management in neuromuscular disorders: Recommendations for future research. Disability and Rehabilitation. Assistive Technology. 2012; 7(6):437-49. [DOI:10.3109/17483107.2012.680940] [PMID]
- Klenow T, Stevens P. Orthotic management of the mangled extremity. In: Pensy RA, Ingari JV, editors. The Mangled Extremity. Cham: Springer; 2021. [Link]
- Harryman SE. Lower-extremity surgery for children with cerebral palsy: Physical therapy management. Physical Therapy. 1992; 72(1):16-24. [DOI:10.1093/ptj/72.1.16] [PMID]
- Takahashi N, Takahashi H, Takahashi O, Ushijima R, Umebayashi R, Nishikawa J, et al. Tone‐Inhibiting Insoles Enhance the Reciprocal Inhibition of Ankle Plantarflexors of Subjects With Hemiparesis After Stroke: An Electromyographic Study. PM & R: The Journal of Injury, Function, and Rehabilitation. 2018; 10(2):168-74. [DOI:10.1016/j.pmrj.2017.07.004] [PMID]
- Maas JC, Dallmeijer AJ, Huijing PA, Brunstrom-Hernandez JE, van Kampen PJ, Jaspers RT, et al. Splint: The efficacy of orthotic management in rest to prevent equinus in children with cerebral palsy, a randomised controlled trial. BMC Pediatrics. 2012; 12:38. [DOI:10.1186/1471-2431-12-38] [PMID]
- Maas J, Dallmeijer A, Huijing P, Brunstrom-Hernandez J, van Kampen P, Bolster E, et al. A randomized controlled trial studying efficacy and tolerance of a knee-ankle-foot orthosis used to prevent equinus in children with spastic cerebral palsy. Clinical Rehabilitation. 2014; 28(10):1025-38. [DOI:10.1177/0269215514542355] [PMID]
- MacFarlane C, Hing W, Orr R. Using the edinburgh visual gait score to compare ankle-foot orthoses, sensorimotor orthoses and barefoot gait pattern in children with cerebral palsy. Children. 2020; 7(6):54. [DOI:10.3390/children7060054] [PMID]
- Lohman M, Goldstein H. Alternative strategies in tone-reducing AFO design. JPO: Journal of Prosthetics and Orthotics. 1993; 5(1):21-4. [DOI:10.1097/00008526-199301000-00003]
- Mol EM, Monbaliu E, Ven M, Vergote M, Prinzie P. The use of night orthoses in cerebral palsy treatment: Sleep disturbance in children and parental burden or not? Research in Developmental Disabilities. 2012; 33(2):341-9. [DOI:10.1016/j.ridd.2011.10.026] [PMID]
- Wayte S, McCaughey E, Holley S, Annaz D, Hill CM. Sleep problems in children with cerebral palsy and their relationship with maternal sleep and depression. Acta Paediatrica. 2012; 101(6):618-23. [DOI:10.1111/j.1651-2227.2012.02603.x] [PMID]
- Lélis AL, Cardoso MV, Hall WA. Sleep disorders in children with cerebral palsy: An integrative review. Sleep Medicine Reviews. 2016; 30:63-71. [DOI:10.1016/j.smrv.2015.11.008] [PMID]
- Kheder A, Nair KP. Spasticity: Pathophysiology, evaluation and management. Practical Neurology. 2012; 12(5):289-98. [DOI:10.1136/practneurol-2011-000155] [PMID]
- Saffari M, Gholamrezaei A, Saneian H, Attari A, Bruni O. Linguistic validation of the Sleep Disturbance Scale for Children (SDSC) in Iranian children with Persian language. Sleep Medicine. 2014; 15(8):998-1001. [DOI:10.1016/j.sleep.2014.03.021] [PMID]
- Crenshaw S, Herzog R, Castagno P, Richards J, Miller F, Michaloski G, et al. The efficacy of tone-reducing features in orthotics on the gait of children with spastic diplegic cerebral palsy. Journal of Pediatric Orthopaedics. 2000; 20(2):210-6. [DOI:10.1097/01241398-200003000-00015] [PMID]
- Ko J, Woo JH, Her JG. The reliability and concurrent validity of the GMFCS for children with cerebral palsy. Journal of Physical Therapy Science. 2011; 23(2):255-8. [Link]
- Nielsen JB, Crone C, Hultborn H. The spinal pathophysiology of spasticity-from a basic science point of view. Acta Physiologica. 2007; 189(2):171-80. [DOI:10.1111/j.1748-1716.2006.01652.x] [PMID]
- MacFarlane C, Orr R, Hing W. Sensomotoric Orthoses, Ankle-Foot Orthoses, and Children with Cerebral Palsy: The Bigger Picture. Children. 2020; 7(8):82. [DOI:10.3390/children7080082] [PMID]
- Mancini VO, Rudaizky D, Pearcy BTD, Marriner A, Pestell CF, Gomez R, et al. Factor structure of the Sleep Disturbance Scale for Children (SDSC) in those with Attention Deficit and Hyperactivity Disorder (ADHD). Sleep Medicine: X. 2019; 1:100006. [DOI:10.1016/j.sleepx.2019.100006] [PMID]
- Smania N, Picelli A, Munari D, Geroin C, Ianes P, Waldner A, et al. Rehabilitation procedures in the management of spasticity. European Journal of Physical and Rehabilitation Medicine. 2010; 46(3):423-38. [PMID]
- Simard-Tremblay E, Constantin E, Gruber R, Brouillette RT, Shevell M. Sleep in children with cerebral palsy: A review. Journal of Child Neurology. 2011; 26(10):1303-10. [DOI:10.1177/0883073811408902] [PMID]
- Horwood L, Mok E, Li P, Oskoui M, Shevell M, Constantin E. Prevalence of sleep problems and sleep-related characteristics in preschool-and school-aged children with cerebral palsy. Sleep Medicine. 2018; 50:1-6. [DOI:10.1016/j.sleep.2018.05.008] [PMID]
- Newman CJ, O’Regan M, Hensey O. Sleep disorders in children with cerebral palsy. Developmental Medicine and Child Neurology. 2006; 48(7):564-8. [PMID]
Article type: Original Research Articles |
Subject:
Orthosis and Prosthesis
Received: 2023/07/16 | Accepted: 2024/02/17 | Published: 2025/06/1
Received: 2023/07/16 | Accepted: 2024/02/17 | Published: 2025/06/1
Send email to the article author








