Volume 22, Issue 4 (December 2024)
Iranian Rehabilitation Journal 2024, 22(4): 583-594 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ali H M, Sulaiman S K, Bello U M, Muhammad A S, Ado F M, Maina H A, et al . Telerehabilitation in Promoting Home-based Upper Extremity Exercises Among Stroke Survivors: A Pilot Study. Iranian Rehabilitation Journal 2024; 22 (4) :583-594
URL: http://irj.uswr.ac.ir/article-1-2080-en.html
URL: http://irj.uswr.ac.ir/article-1-2080-en.html
Hafsat Maina Ali1 

 , Surajo Kamilu Sulaiman *2
, Surajo Kamilu Sulaiman *2 

 , Umar Muhammad Bello3
, Umar Muhammad Bello3 

 , Abdullahi Salisu Muhammad4
, Abdullahi Salisu Muhammad4 

 , Fatima Mahmud Ado5
, Fatima Mahmud Ado5 

 , Hassan Ali Maina6
, Hassan Ali Maina6 

 , Hussaina Abubakar Jalo7
, Hussaina Abubakar Jalo7 
 , Mohammed Usman Ali8
, Mohammed Usman Ali8 

 , Ismail Muhammad Bello9
, Ismail Muhammad Bello9 

 , Halima Maina10
, Halima Maina10 




 , Surajo Kamilu Sulaiman *2
, Surajo Kamilu Sulaiman *2 

 , Umar Muhammad Bello3
, Umar Muhammad Bello3 

 , Abdullahi Salisu Muhammad4
, Abdullahi Salisu Muhammad4 

 , Fatima Mahmud Ado5
, Fatima Mahmud Ado5 

 , Hassan Ali Maina6
, Hassan Ali Maina6 

 , Hussaina Abubakar Jalo7
, Hussaina Abubakar Jalo7 
 , Mohammed Usman Ali8
, Mohammed Usman Ali8 

 , Ismail Muhammad Bello9
, Ismail Muhammad Bello9 

 , Halima Maina10
, Halima Maina10 


1- Department of Physiotherapy, Bayero University, Kano, Nigeria.
2- Department of Physiotherapy, Faculty of Applied Science, Tishk International University, Erbil, Iraq.
3- Department of Paramedicine and Physiotherapy, Glasgow Caledonia University, Glasgow, United Kingdom.
4- Department of Physiotherapy, Yobe State University Teaching Hospital, Damaturu, Nigeria.
5- Department of Physiotherapy, Yobe State Specialist Hospital, Damaturu, Nigeria.
6- Department of Anesthesia, Modibbo Adama University Teaching Hospital, Madumari, Nigeria.
7- Department of Pediatrics, Yobe State Specialist Hospital, Damaturu, Nigeria.
8- Department of Medical Rehabilitation, University of Maiduguri, Maiduguri, Nigeria.
9- Department of Pediatrics, Khalifa Sheikh Isyaka Rabiu Paediatric Hospital, Kano, Nigeria.
10- Department of Nursing, Shehu Sule College of Nursing and Midwifery, Damaturu, Nigeria.
2- Department of Physiotherapy, Faculty of Applied Science, Tishk International University, Erbil, Iraq.
3- Department of Paramedicine and Physiotherapy, Glasgow Caledonia University, Glasgow, United Kingdom.
4- Department of Physiotherapy, Yobe State University Teaching Hospital, Damaturu, Nigeria.
5- Department of Physiotherapy, Yobe State Specialist Hospital, Damaturu, Nigeria.
6- Department of Anesthesia, Modibbo Adama University Teaching Hospital, Madumari, Nigeria.
7- Department of Pediatrics, Yobe State Specialist Hospital, Damaturu, Nigeria.
8- Department of Medical Rehabilitation, University of Maiduguri, Maiduguri, Nigeria.
9- Department of Pediatrics, Khalifa Sheikh Isyaka Rabiu Paediatric Hospital, Kano, Nigeria.
10- Department of Nursing, Shehu Sule College of Nursing and Midwifery, Damaturu, Nigeria.
Keywords: Telerehabilitation (TR), Upper extremity, Stroke rehabilitation, Home program, Self-management
Full-Text [PDF 558 kb]
(1178 Downloads)
| Abstract (HTML) (3862 Views)
Full-Text: (454 Views)
Introduction
Stroke ranks as a prominent contributor to global disability [1]. Worldwide, around 15 million individuals experience a stroke annually, leading to nearly 6 million fatalities due to direct effects or other complications of the stroke [2-4]. Thus, stroke continues to be a significant factor contributing to global mortality and morbidity [5]. The most common deficit after stroke is seen in the motor system, affecting more than 80% of patients [6]. Only a small number of patients achieve complete recovery from upper limb paralysis following a stroke. At the same time, the majority demonstrate persistent impairments, leading to limitations in activities, restrictions, and participation and decreased life satisfaction, quality of life (QOL), and overall welfare [7, 8].
In most developing countries that lack functional community rehabilitation services, a continuous hospital visit is required for therapeutic services in the rehabilitation of post-stroke patients, especially physiotherapy and occupational therapy for functional recovery. Many outpatients do not receive adequate treatment due to barriers such as problems with commuting, dependence on caregivers, limited outpatient services, poor social support systems, and limited finances and health insurance coverage [9, 10]. It is crucial to continue intensive physiotherapy to improve the upper extremity function after hospitalization [11, 12]. Stroke survivors could benefit from a system that allows health professionals to provide rehabilitation services from a remote location. Moreover, the COVID-19 pandemic left many post-stroke survivors without adequate rehabilitation services due to a shortage of staff and measures to curtail the spread of the disease, among other reasons [13]. The high burden of stroke and inadequate rehabilitation services prompt the need to develop and evaluate new strategies, such as the use of telerehabilitation (TR) [14].
TR refers to the delivery of health services via electronic communication (websites, telephone, mobile apps) geared towards enhancing an individual's health, offering education and services, and providing equal access to geographically remote patients who are physically and economically disadvantaged [9]. Existing TR studies indicate that individuals diagnosed with stroke who received their rehabilitation using a smartphone and or videoconferencing show an equal or even better improvement in their health status compared to those who received only usual care [15-17]. A recent study consistently indicated significant improvement in upper limb function due to intensive exercise programs delivered via TR [18]. Studies have also shown positive outcomes and high patient satisfaction with TR across various healthcare conditions in developed countries [19-22]. Further, evidence has demonstrated that TR is safe and feasible among community-dwelling persons with stroke [23-25].
Most of the available evidence on the utility and feasibility of TR was reported from developed countries that have high technological advancement and may not be extrapolated to resource-poor settings like Nigeria. Despite the potential benefits of TR, there is a shortage of evidence on its utility and acceptability in Nigeria. Thus, this study aimed to provide evidence on the effectiveness and feasibility of TR in promoting home-based upper extremity exercises among stroke survivors in Nigeria.
Materials and Methods
Study design
This research employed a parallel two-arm design with blinded outcome assessors. All the study participants duly signed the informed consent form, and all research procedures adhered to the principles outlined in the Declaration of Helsinki [26, 27].
Participants and recruitment
Participants were stroke survivors from outpatient physiotherapy clinics in Nigeria. The inclusion criteria were as follows: An episode of a cerebrovascular accident leading to one-sided hemiplegia or hemiparesis, age range between 40 to 65 years on the day of data collection, a stroke of ≥6 months, stable cognitive functioning (Montreal cognitive assessment [MoCA] ≥26 points) [28], and stable clinical condition [29]. The exclusion criteria were as follows: Cognitive impairment such as apraxia, neglect and language disturbances interfering with verbal comprehension, disturbed unaffected upper limb function, medical complications, and other problems possibly contra-indicating self-directed exercise at home [30]. The sample size was estimated based on a study where 7 adults with stroke participated in home-based TR [19].
Intervention (TR group)
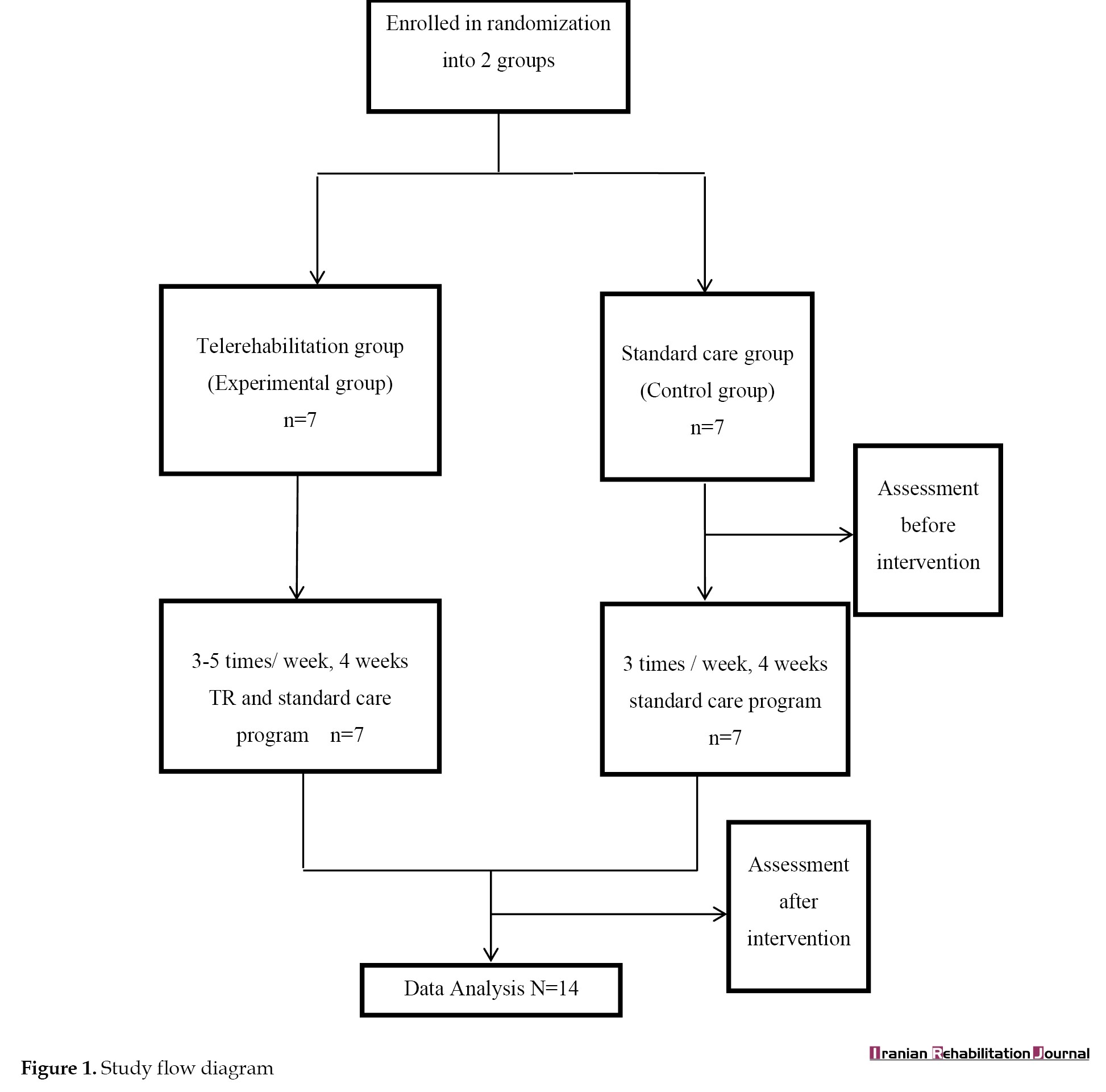
Participants in the intervention group performed a 4-week rehabilitation program that was task-oriented, individually customized, and intensive. For each participant, a physiotherapist prescribed an individualized exercise program (home program) with appropriate mode, frequency, duration, and intensity (number of repetitions and challenges involved), depending on the traits of the participant's impairments noted at the initial assessments [31]. During the study, the rehabilitation of the participants progressed through an exercise encompassing fundamental elements of reaching, grasping, holding, and manipulation, advancing in difficulty levels. An assigned physiotherapist moderated the exercises via audio calls. Audio telephone calls were employed to address complaints and queries, excluding the review and adjustment of the exercise prescription. Adjustments to the exercises prescribed were done during the usual hospital visits (usual care). Physiotherapist-patient contact via TR services was limited to 3-5 times a week. Participants were required to engage in their exercise program by using any exercise devices prescribed in conjunction with the guidance from the information sheet outlining their personalized exercise regimen. The patients were asked to return to the hospital if adverse clinical events occurred at home during the exercises. Assistance was provided by the caregivers of participants requiring support with their home program. In addition to the TR, participants in the intervention group received standard care involving regular outpatient physiotherapy visits (Figure 1).
Control group (standard care only)
Participants in the control group exclusively received standard care, consisting of usual hospital visits to physiotherapy outpatient clinics as needed and regular home programs without physiotherapists' contact via TR services.
Outcome measures
Primary outcome
Fugl-Meyer upper extremity motor assessment (FMA-UE)
The FMA is an observer-administered tool comprising 5 domains: Motor function (upper and lower extremity sub-domains), balance, joint pain, joint range of motion, and sensory function [32]. The scoring is done using a 3-point ordinal scale [32]. FMA-UE subscore for the upper arm is 36. The minimal clinically significant difference of the FMA-UE is 5.6 [33]. The FMA is psychometrically sound, with excellent scores for internal consistency (Cronbach α=0.94 to 0.98) [32]. The FMA exhibited a notable ceiling effect, with over 44.4% of subjects attaining the highest score. Additionally, the FMA demonstrates low to moderate validity (The Spearman rho ranging from 0.29 to 0.53) and low to moderate responsiveness (standardized response mean ranging from 0.27 to 0.67) [33].
Wolf motor function test (WMFT)
The Wolf motor function test (WMFT) represents a numerical measure of upper extremity function and motor deficiency in individuals affected by stroke or traumatic brain injury [34]. It consists of three parts (time, functional ability, and strength), including 15 functional-based tasks and two strength-based tasks. The WMFT time allows 120 seconds per task. It uses a 6-point ordinal scale of 0 to 5, with a maximum of 75 scores, where lower scores indicate a lower functioning level [35]. The inter-rater reliability of WMFT is excellent, with a score of 0.97. Additionally, the internal consistency measured by Cronbach α is high at 0.92. Furthermore, the stability of the WMFT is also deemed excellent [34].
Secondary outcome
World Health Organization (WHO) quality of life BREF (WHOQOL-BREF)
The WHOQOL-BREF is commonly used to measure the QOL in healthy and diseased populations [36] with 26 items [37]. Items number one and two ask about general health and overall QOL, whereas the remaining 24 questions that follow are categorized based on QOL domains [38]: Physical health, psychological, social relationship, and environment [39]. Each question is scored using a 5-point Likert scale, and domain scores are then converted to a scale of 0-100 points [37]. A score of 100 indicates better QOL, while 0 indicates a low QOL based on the scoring guideline [40]. WHQOL-BREF has shown moderate to high internal consistency as indicated by Cronbach α, ranging from 0.66 to 0.80, and excellent constructs validity (r=0.92) when correlated with scores from WHOQOL-100 [38].
Barthel index (BI)
Barthel index (BI) is a commonly employed standardized tool by clinicians and researchers to evaluate disability in activities of daily living (ADL). It encompasses basic daily living (ADL) activities such as feeding, grooming, bathing, dressing, bowel and bladder care, toilet use, ambulation, transfers, and stair climbing. The total score on the BI spans from 0 to 20, with elevated scores indicating greater levels of functionality [41]. The BI is psychometrically sound among raters (kappa value range: 0.53-0.94, ICC=0.94) and has strong internal consistency (alpha=0.89 to 0.90). ADL was measured at baseline and four weeks [41].
Feasibility
Adherence
The patient's adherence was assessed by monitoring the frequency of calls made to them 3 to 5 times per week. These calls allowed tracking of the patient's attendance at each single session.
Acceptability
Acceptability of the intervention was measured using a logbook to monitor adherence (acceptability). The TR group was asked to tick the logbook after each treatment session following the phone call.
Satisfaction
The satisfaction of the intervention was evaluated subjectively using closed-ended questions. At the end of the fourth week during the data collection, stroke survivors were inquired about their contentment with the intervention received at the physiotherapy clinic with a yes and no answer.
Procedure
Physiotherapists at the recruitment sites screened the patients for eligibility. The eligible patients were duly informed of the study rationale and procedure and were enlightened about the aim of the research. The patients provided written informed consent, and their confidentiality was guaranteed. Concealment of allocation was achieved using numbered, sealed, and opaque envelopes during the group allocation. This process resulted in allocating the participants into the intervention (TR+standard care) and control (standard care only) groups. The research member involved in the randomization process did not participate in administering the interventions and assessment process. Blinding was not possible in the study due to the nature of the intervention, making the researchers and participants fully aware of their assigned treatment groups. Two blinded outcome assessors conducted the baseline evaluation, which consisted of demographic characteristics (age, gender, educational level) and clinical variables (laterality, type, duration of stroke, and cognitive status). The outcomes were motor impairment, motor function, QoL, and ADLs, which were assessed using the FMA-UE, WMFT, WHOQOL-BREF, and BI, respectively. The two blinded outcome assessors reassessed the participants 4 weeks after completion of the intervention course using the same outcome measures.
Data analysis
The statistical analysis was performed using SPSS software, version 20 (SPSS Inc., Chicago, IL, USA), using a variety of tests as appropriate. Demographic characteristics of the participants (age, gender, type of stroke, affected side, level of education, and time since stroke) were summarized using descriptive statistics. To ensure homogeneity among the groups at baseline, independent t-test and chi-square test were used to analyze differences in the clinical and sociodemographic data among the two groups. An independent t-test was used to measure the effect of the treatment on upper limb function, QOL, and ADL. A significance level of P<0.05 was employed to denote statistical significance. The feasibility analysis involves examining several factors, including assessing the frequency of logbook entries, tallying the responses to close-ended questions, distinguishing between 'yes' and 'no' answers, and quantifying and analyzing the number of responsive phone calls made to stroke survivors. This information was used to calculate the corresponding percentages.
Results
Demographics of participants
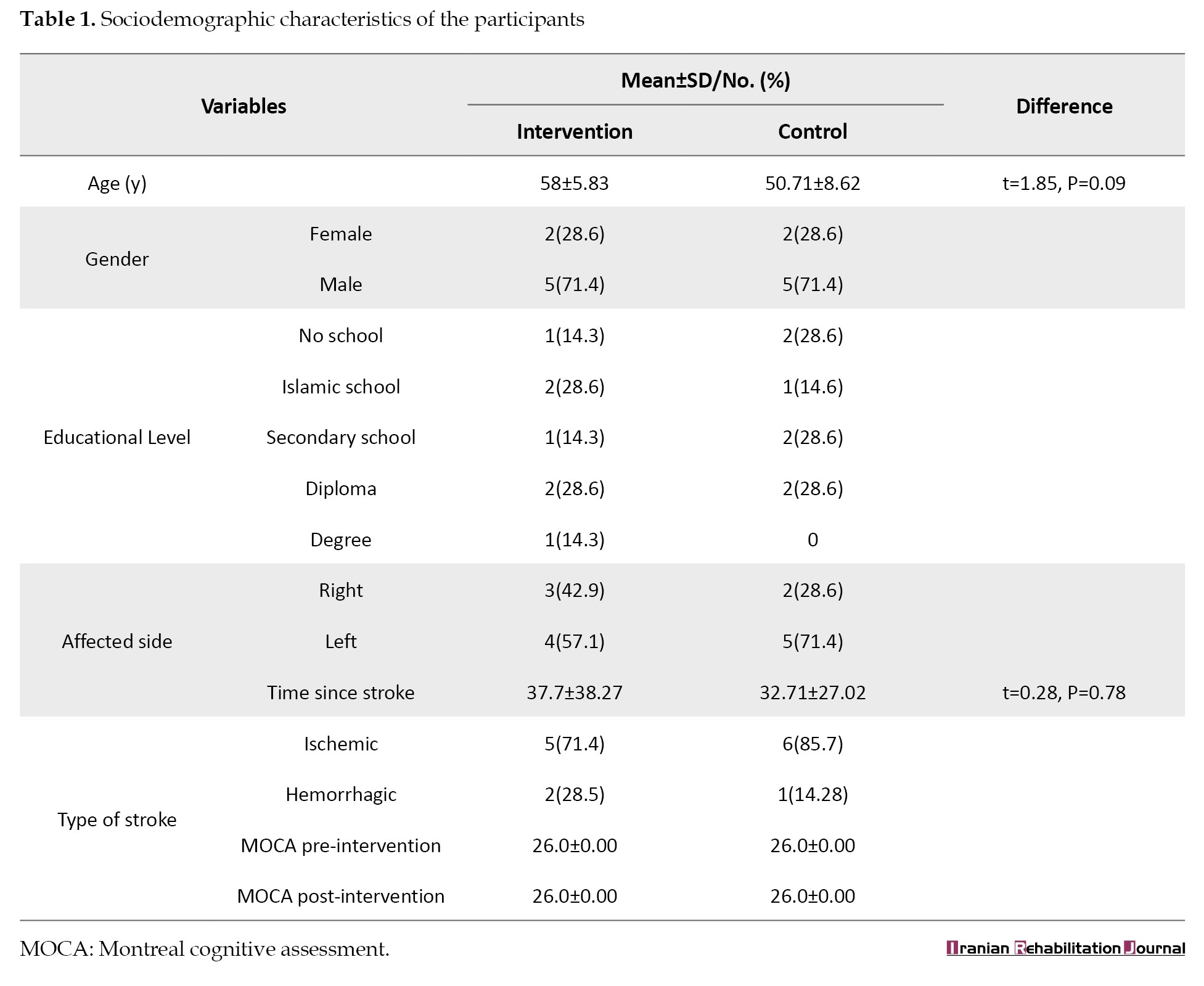
Fourteen stroke survivors (10 males) with a mean (SD) age of 50 (8.2) years participated in the study. Seven participants were randomized to the intervention (5 males) and 7 to the usual care (5 males) group. Both groups were comparable in their ages at baseline (P=0.09). Baseline comparison revealed that no difference was observed between the groups regarding the educational level, time since stroke, and paretic side (P> 0.05). The majority of the participants had ischemic stroke, which accounts for 71.4% of the TR group and 85.7% in the standard care (Table 1).
No adverse events were reported by any participant during the study.
Effect of TR on upper limb function assessed using FMA–UE
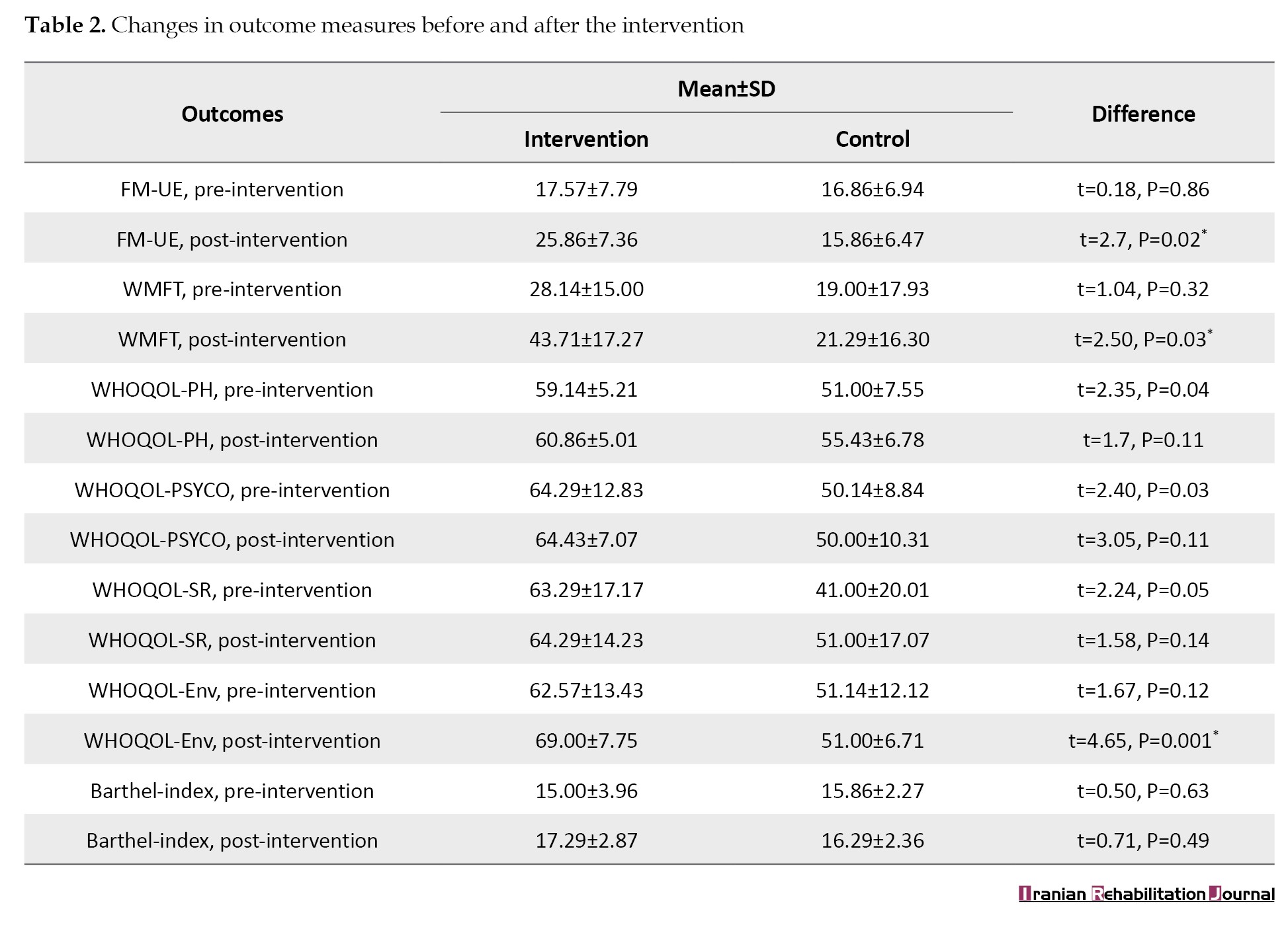
The mean FMA-UE of the participants in the two study groups was not significantly different at baseline (P=0.86) but showed significant (P=0.02) differences after the TR intervention, with the TR+standard care showing greater change (17.57 to 25.86) relative to the baseline, compared to the control group (16.86 to 15.86)(Table 2).
Effect of TR on upper limb function assessed using WMFT
The mean WMFT of the participants in the two study groups was not significantly different at baseline (P=0.32) but showed significant (P=0.03) differences after the TR intervention, with the TR+standard care showing greater change (28.14 to 43.71) relative to the baseline, compared to the control group (19.00 to 21.29) (Table 2).
Effect of TR on QOL
The mean WHOQOL environment, WHOQOL physical health, WHOQOL psychological health, and WHOQOL social relationship of the participants in the two study groups was not significantly different at baseline. However, the WHOQOL environment showed significant (P=0.001) differences after the TR intervention, with the TR+standard care showing greater change (62.57±13.43) relative to the baseline, compared to the control group (69.00±7.75) (Table 2).
Effect of TR on ADL
The mean BI of the participants in the two study groups was not significantly different at baseline (P=0.63); it also showed no significant (P=0.49) difference after the TR intervention (Table 2).
Feasibility
Adherence
No participant withdrew from the TR intervention group. Thus, we recorded high adherence to the intervention (100%).
Acceptability
TR was acceptable to the participants; they practiced their exercise program as prescribed, indicating high acceptability (100%).
Satisfaction
The TR group participants reported higher satisfaction with the intervention compared to those in the usual care group (100%).
Discussion
This study aimed to find preliminary evidence on the feasibility and influence of audio-based TR in promoting home-based upper extremity exercises among stroke patients. Findings from this study show that a 4-week TR intervention is feasible and effective, producing substantial gains in arm motor function and QOL among stroke patients.
The primary functional outcomes, FMA-UE and WMFT, improved significantly after the intervention, possibly due to improved brain plasticity and the restoration of functional capabilities among the participants [22, 42]. There was a substantial improvement in the environment domain of the WHOQOL-BREF, which could be attributed to the influence of the environment where the patients received the TR treatment [36]. However, there was no significant improvement in physical health, psychological, and social relationships domains of WHOQOL-BREF following the 4 weeks intervention [36]. Multiple studies have shown that familiar environments can improve rehabilitation outcomes by promoting meaningful task-specific training, providing a sense of control, increasing confidence, and enhancing skills [43]. Data from this study showed no evidence of a beneficial effect of TR when compared with usual care on ADLs. Presumably, the patients had already learned to independently manage their daily needs without using their affected arm [44]. The positive intervention changes observed in motor function and QOL in the present study are good indicators; however, only a randomized trial can provide substantial evidence of the effectiveness of the TR intervention [45, 46]. The patients expressed satisfaction with the intervention and were motivated to remain in the program because the intervention resulted in positive outcomes such as improved physical functioning and QoL (Q). The study findings indicate that using TR potentially enhances motor function among stroke survivors [47].
The result of this study shows that the motor function of patients in the TR group was significantly better than that of patients in the standard care group. This finding is consistent with the previous findings reported by Cramer and colleagues, who observed significant improvements in physical function in post-stroke patients who participated in a video-based TR [48]. Similarly, TR was shown to be effective in promoting motor function restoration in stroke patients' upper extremities [49]. A notable improvement in physical performance measured with the FMA-UE was reported [49].
On the other hand, the present study shows no significant difference in ADLs between the TR and standard care group. These findings align with the study outcomes by Chumbler et al. and Forducey et al., who assessed independence in ADLs after TR intervention [50, 51]. Both studies observed no notable difference between the intervention and control groups in ADLs [50, 51]. Moreover, this study documented a substantial enhancement in the environmental domain of QOL. However, this finding is partly consistent with the literature, indicating that TR significantly improves all domains of QOL measured with WHOQOL-BREF [52].
Furthermore, previous studies have reported that TR was feasible and well-received by stroke patients [49, 52, 53]. The findings of the present study corroborate the feasibility of TR, as reported in the literature. There is a shortage of studies that focus specifically on the use of audio-based TR in promoting upper limb motor function among stroke patients, thus limiting comparison of the findings from the present study.
This study is not without limitations. We employed a feasibility design with an insufficient sample size, thus limiting the applicability of the findings. Hence, studies with a more robust design and a larger sample size are recommended. Although the study primarily concentrated on the upper extremity of the post-stroke patient, several other areas of impairment could benefit from TR, such as deficits in the lower limb. The majority of the participants are middle-aged males with ischemic stroke; thus, the findings of the study should be interpreted with caution. Hence, future studies with representative samples are warranted. The TR mode of intervention would indisputably reduce clinical visits and waiting time. On the other hand, we believe TR may not be appropriate for individuals with severe impairments due to potential hindrances caused by worsened disabilities, such as spasticity and contracture, that may negatively affect motor skills [53].
However, the study has some strengths, as follows. The intervention facilitated two-way communication between physiotherapists and patients, thus providing a means for proper communication. The assessors were blinded, and the participants were randomly assigned to minimize bias and improve the study's validity. The audio-based TR contributes to a notable enhancement in functioning during the early stage of recovery. The TR recipients found it acceptable, completing almost 100% of therapy sessions with no reports of serious adverse events related to treatment.
Conclusion
This study revealed that TR intervention is feasible and shows significant promise in enhancing upper limb motor function and QOL among adult stroke survivors. Therefore, TR can be considered a complementary intervention to the conventional in-person approach to upper extremity rehabilitation among stroke patients and promote access and affordability of rehabilitation services. Further, this study suggests that using TR to deliver upper limb rehabilitation could be superior to conventional methods in improving motor function and QOL among stroke patients. However, further studies in the form of randomized controlled trials with larger representative samples are warranted to validate the effectiveness of the TR intervention.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Ministry of Health and Human Services (Code: MOH/GEN/747/001/01) granted the study's approval. The study was registered at Pan African Clinical Trials (Code: PACTR202301605291913).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and Methodology: Hafsat Maina Ali, Surajo Kamilu Sulaiman, and Umar Muhammad Bello; Supervision and data analysis: Surajo Kamilu Sulaiman and Umar Muhammad Bello; Data collection: Abdullahi Salisu Muhammad, Fatima Mahmud Ado, Hassan Ali Maina, Hussaina Abubakar Jalo, Mohammed Usman Ali, Ismail Muhammad Bello, and Halima Maina; Writing the original draft: Hafsat Maina Ali, Abdullahi Salisu Muhammad, Fatima Mahmud Ado, Hassan Ali Maina, Hussaina Abubakar Jalo, Mohammed Usman Ali, Ismail Muhammad Bello, and Halima Maina; Review and editing: Surajo Kamilu Sulaiman and Umar Muhammad Bello.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to Fatima Sa’ad for contributing to the recruitment of the participants and Stephen Chinonso Ogbunamiri for providing technical support.
Stroke ranks as a prominent contributor to global disability [1]. Worldwide, around 15 million individuals experience a stroke annually, leading to nearly 6 million fatalities due to direct effects or other complications of the stroke [2-4]. Thus, stroke continues to be a significant factor contributing to global mortality and morbidity [5]. The most common deficit after stroke is seen in the motor system, affecting more than 80% of patients [6]. Only a small number of patients achieve complete recovery from upper limb paralysis following a stroke. At the same time, the majority demonstrate persistent impairments, leading to limitations in activities, restrictions, and participation and decreased life satisfaction, quality of life (QOL), and overall welfare [7, 8].
In most developing countries that lack functional community rehabilitation services, a continuous hospital visit is required for therapeutic services in the rehabilitation of post-stroke patients, especially physiotherapy and occupational therapy for functional recovery. Many outpatients do not receive adequate treatment due to barriers such as problems with commuting, dependence on caregivers, limited outpatient services, poor social support systems, and limited finances and health insurance coverage [9, 10]. It is crucial to continue intensive physiotherapy to improve the upper extremity function after hospitalization [11, 12]. Stroke survivors could benefit from a system that allows health professionals to provide rehabilitation services from a remote location. Moreover, the COVID-19 pandemic left many post-stroke survivors without adequate rehabilitation services due to a shortage of staff and measures to curtail the spread of the disease, among other reasons [13]. The high burden of stroke and inadequate rehabilitation services prompt the need to develop and evaluate new strategies, such as the use of telerehabilitation (TR) [14].
TR refers to the delivery of health services via electronic communication (websites, telephone, mobile apps) geared towards enhancing an individual's health, offering education and services, and providing equal access to geographically remote patients who are physically and economically disadvantaged [9]. Existing TR studies indicate that individuals diagnosed with stroke who received their rehabilitation using a smartphone and or videoconferencing show an equal or even better improvement in their health status compared to those who received only usual care [15-17]. A recent study consistently indicated significant improvement in upper limb function due to intensive exercise programs delivered via TR [18]. Studies have also shown positive outcomes and high patient satisfaction with TR across various healthcare conditions in developed countries [19-22]. Further, evidence has demonstrated that TR is safe and feasible among community-dwelling persons with stroke [23-25].
Most of the available evidence on the utility and feasibility of TR was reported from developed countries that have high technological advancement and may not be extrapolated to resource-poor settings like Nigeria. Despite the potential benefits of TR, there is a shortage of evidence on its utility and acceptability in Nigeria. Thus, this study aimed to provide evidence on the effectiveness and feasibility of TR in promoting home-based upper extremity exercises among stroke survivors in Nigeria.
Materials and Methods
Study design
This research employed a parallel two-arm design with blinded outcome assessors. All the study participants duly signed the informed consent form, and all research procedures adhered to the principles outlined in the Declaration of Helsinki [26, 27].
Participants and recruitment
Participants were stroke survivors from outpatient physiotherapy clinics in Nigeria. The inclusion criteria were as follows: An episode of a cerebrovascular accident leading to one-sided hemiplegia or hemiparesis, age range between 40 to 65 years on the day of data collection, a stroke of ≥6 months, stable cognitive functioning (Montreal cognitive assessment [MoCA] ≥26 points) [28], and stable clinical condition [29]. The exclusion criteria were as follows: Cognitive impairment such as apraxia, neglect and language disturbances interfering with verbal comprehension, disturbed unaffected upper limb function, medical complications, and other problems possibly contra-indicating self-directed exercise at home [30]. The sample size was estimated based on a study where 7 adults with stroke participated in home-based TR [19].
Intervention (TR group)
Participants in the intervention group performed a 4-week rehabilitation program that was task-oriented, individually customized, and intensive. For each participant, a physiotherapist prescribed an individualized exercise program (home program) with appropriate mode, frequency, duration, and intensity (number of repetitions and challenges involved), depending on the traits of the participant's impairments noted at the initial assessments [31]. During the study, the rehabilitation of the participants progressed through an exercise encompassing fundamental elements of reaching, grasping, holding, and manipulation, advancing in difficulty levels. An assigned physiotherapist moderated the exercises via audio calls. Audio telephone calls were employed to address complaints and queries, excluding the review and adjustment of the exercise prescription. Adjustments to the exercises prescribed were done during the usual hospital visits (usual care). Physiotherapist-patient contact via TR services was limited to 3-5 times a week. Participants were required to engage in their exercise program by using any exercise devices prescribed in conjunction with the guidance from the information sheet outlining their personalized exercise regimen. The patients were asked to return to the hospital if adverse clinical events occurred at home during the exercises. Assistance was provided by the caregivers of participants requiring support with their home program. In addition to the TR, participants in the intervention group received standard care involving regular outpatient physiotherapy visits (Figure 1).
Control group (standard care only)
Participants in the control group exclusively received standard care, consisting of usual hospital visits to physiotherapy outpatient clinics as needed and regular home programs without physiotherapists' contact via TR services.
Outcome measures
Primary outcome
Fugl-Meyer upper extremity motor assessment (FMA-UE)
The FMA is an observer-administered tool comprising 5 domains: Motor function (upper and lower extremity sub-domains), balance, joint pain, joint range of motion, and sensory function [32]. The scoring is done using a 3-point ordinal scale [32]. FMA-UE subscore for the upper arm is 36. The minimal clinically significant difference of the FMA-UE is 5.6 [33]. The FMA is psychometrically sound, with excellent scores for internal consistency (Cronbach α=0.94 to 0.98) [32]. The FMA exhibited a notable ceiling effect, with over 44.4% of subjects attaining the highest score. Additionally, the FMA demonstrates low to moderate validity (The Spearman rho ranging from 0.29 to 0.53) and low to moderate responsiveness (standardized response mean ranging from 0.27 to 0.67) [33].
Wolf motor function test (WMFT)
The Wolf motor function test (WMFT) represents a numerical measure of upper extremity function and motor deficiency in individuals affected by stroke or traumatic brain injury [34]. It consists of three parts (time, functional ability, and strength), including 15 functional-based tasks and two strength-based tasks. The WMFT time allows 120 seconds per task. It uses a 6-point ordinal scale of 0 to 5, with a maximum of 75 scores, where lower scores indicate a lower functioning level [35]. The inter-rater reliability of WMFT is excellent, with a score of 0.97. Additionally, the internal consistency measured by Cronbach α is high at 0.92. Furthermore, the stability of the WMFT is also deemed excellent [34].
Secondary outcome
World Health Organization (WHO) quality of life BREF (WHOQOL-BREF)
The WHOQOL-BREF is commonly used to measure the QOL in healthy and diseased populations [36] with 26 items [37]. Items number one and two ask about general health and overall QOL, whereas the remaining 24 questions that follow are categorized based on QOL domains [38]: Physical health, psychological, social relationship, and environment [39]. Each question is scored using a 5-point Likert scale, and domain scores are then converted to a scale of 0-100 points [37]. A score of 100 indicates better QOL, while 0 indicates a low QOL based on the scoring guideline [40]. WHQOL-BREF has shown moderate to high internal consistency as indicated by Cronbach α, ranging from 0.66 to 0.80, and excellent constructs validity (r=0.92) when correlated with scores from WHOQOL-100 [38].
Barthel index (BI)
Barthel index (BI) is a commonly employed standardized tool by clinicians and researchers to evaluate disability in activities of daily living (ADL). It encompasses basic daily living (ADL) activities such as feeding, grooming, bathing, dressing, bowel and bladder care, toilet use, ambulation, transfers, and stair climbing. The total score on the BI spans from 0 to 20, with elevated scores indicating greater levels of functionality [41]. The BI is psychometrically sound among raters (kappa value range: 0.53-0.94, ICC=0.94) and has strong internal consistency (alpha=0.89 to 0.90). ADL was measured at baseline and four weeks [41].
Feasibility
Adherence
The patient's adherence was assessed by monitoring the frequency of calls made to them 3 to 5 times per week. These calls allowed tracking of the patient's attendance at each single session.
Acceptability
Acceptability of the intervention was measured using a logbook to monitor adherence (acceptability). The TR group was asked to tick the logbook after each treatment session following the phone call.
Satisfaction
The satisfaction of the intervention was evaluated subjectively using closed-ended questions. At the end of the fourth week during the data collection, stroke survivors were inquired about their contentment with the intervention received at the physiotherapy clinic with a yes and no answer.
Procedure
Physiotherapists at the recruitment sites screened the patients for eligibility. The eligible patients were duly informed of the study rationale and procedure and were enlightened about the aim of the research. The patients provided written informed consent, and their confidentiality was guaranteed. Concealment of allocation was achieved using numbered, sealed, and opaque envelopes during the group allocation. This process resulted in allocating the participants into the intervention (TR+standard care) and control (standard care only) groups. The research member involved in the randomization process did not participate in administering the interventions and assessment process. Blinding was not possible in the study due to the nature of the intervention, making the researchers and participants fully aware of their assigned treatment groups. Two blinded outcome assessors conducted the baseline evaluation, which consisted of demographic characteristics (age, gender, educational level) and clinical variables (laterality, type, duration of stroke, and cognitive status). The outcomes were motor impairment, motor function, QoL, and ADLs, which were assessed using the FMA-UE, WMFT, WHOQOL-BREF, and BI, respectively. The two blinded outcome assessors reassessed the participants 4 weeks after completion of the intervention course using the same outcome measures.
Data analysis
The statistical analysis was performed using SPSS software, version 20 (SPSS Inc., Chicago, IL, USA), using a variety of tests as appropriate. Demographic characteristics of the participants (age, gender, type of stroke, affected side, level of education, and time since stroke) were summarized using descriptive statistics. To ensure homogeneity among the groups at baseline, independent t-test and chi-square test were used to analyze differences in the clinical and sociodemographic data among the two groups. An independent t-test was used to measure the effect of the treatment on upper limb function, QOL, and ADL. A significance level of P<0.05 was employed to denote statistical significance. The feasibility analysis involves examining several factors, including assessing the frequency of logbook entries, tallying the responses to close-ended questions, distinguishing between 'yes' and 'no' answers, and quantifying and analyzing the number of responsive phone calls made to stroke survivors. This information was used to calculate the corresponding percentages.
Results
Demographics of participants
Fourteen stroke survivors (10 males) with a mean (SD) age of 50 (8.2) years participated in the study. Seven participants were randomized to the intervention (5 males) and 7 to the usual care (5 males) group. Both groups were comparable in their ages at baseline (P=0.09). Baseline comparison revealed that no difference was observed between the groups regarding the educational level, time since stroke, and paretic side (P> 0.05). The majority of the participants had ischemic stroke, which accounts for 71.4% of the TR group and 85.7% in the standard care (Table 1).

No adverse events were reported by any participant during the study.
Effect of TR on upper limb function assessed using FMA–UE
The mean FMA-UE of the participants in the two study groups was not significantly different at baseline (P=0.86) but showed significant (P=0.02) differences after the TR intervention, with the TR+standard care showing greater change (17.57 to 25.86) relative to the baseline, compared to the control group (16.86 to 15.86)(Table 2).

Effect of TR on upper limb function assessed using WMFT
The mean WMFT of the participants in the two study groups was not significantly different at baseline (P=0.32) but showed significant (P=0.03) differences after the TR intervention, with the TR+standard care showing greater change (28.14 to 43.71) relative to the baseline, compared to the control group (19.00 to 21.29) (Table 2).
Effect of TR on QOL
The mean WHOQOL environment, WHOQOL physical health, WHOQOL psychological health, and WHOQOL social relationship of the participants in the two study groups was not significantly different at baseline. However, the WHOQOL environment showed significant (P=0.001) differences after the TR intervention, with the TR+standard care showing greater change (62.57±13.43) relative to the baseline, compared to the control group (69.00±7.75) (Table 2).
Effect of TR on ADL
The mean BI of the participants in the two study groups was not significantly different at baseline (P=0.63); it also showed no significant (P=0.49) difference after the TR intervention (Table 2).
Feasibility
Adherence
No participant withdrew from the TR intervention group. Thus, we recorded high adherence to the intervention (100%).
Acceptability
TR was acceptable to the participants; they practiced their exercise program as prescribed, indicating high acceptability (100%).
Satisfaction
The TR group participants reported higher satisfaction with the intervention compared to those in the usual care group (100%).
Discussion
This study aimed to find preliminary evidence on the feasibility and influence of audio-based TR in promoting home-based upper extremity exercises among stroke patients. Findings from this study show that a 4-week TR intervention is feasible and effective, producing substantial gains in arm motor function and QOL among stroke patients.
The primary functional outcomes, FMA-UE and WMFT, improved significantly after the intervention, possibly due to improved brain plasticity and the restoration of functional capabilities among the participants [22, 42]. There was a substantial improvement in the environment domain of the WHOQOL-BREF, which could be attributed to the influence of the environment where the patients received the TR treatment [36]. However, there was no significant improvement in physical health, psychological, and social relationships domains of WHOQOL-BREF following the 4 weeks intervention [36]. Multiple studies have shown that familiar environments can improve rehabilitation outcomes by promoting meaningful task-specific training, providing a sense of control, increasing confidence, and enhancing skills [43]. Data from this study showed no evidence of a beneficial effect of TR when compared with usual care on ADLs. Presumably, the patients had already learned to independently manage their daily needs without using their affected arm [44]. The positive intervention changes observed in motor function and QOL in the present study are good indicators; however, only a randomized trial can provide substantial evidence of the effectiveness of the TR intervention [45, 46]. The patients expressed satisfaction with the intervention and were motivated to remain in the program because the intervention resulted in positive outcomes such as improved physical functioning and QoL (Q). The study findings indicate that using TR potentially enhances motor function among stroke survivors [47].
The result of this study shows that the motor function of patients in the TR group was significantly better than that of patients in the standard care group. This finding is consistent with the previous findings reported by Cramer and colleagues, who observed significant improvements in physical function in post-stroke patients who participated in a video-based TR [48]. Similarly, TR was shown to be effective in promoting motor function restoration in stroke patients' upper extremities [49]. A notable improvement in physical performance measured with the FMA-UE was reported [49].
On the other hand, the present study shows no significant difference in ADLs between the TR and standard care group. These findings align with the study outcomes by Chumbler et al. and Forducey et al., who assessed independence in ADLs after TR intervention [50, 51]. Both studies observed no notable difference between the intervention and control groups in ADLs [50, 51]. Moreover, this study documented a substantial enhancement in the environmental domain of QOL. However, this finding is partly consistent with the literature, indicating that TR significantly improves all domains of QOL measured with WHOQOL-BREF [52].
Furthermore, previous studies have reported that TR was feasible and well-received by stroke patients [49, 52, 53]. The findings of the present study corroborate the feasibility of TR, as reported in the literature. There is a shortage of studies that focus specifically on the use of audio-based TR in promoting upper limb motor function among stroke patients, thus limiting comparison of the findings from the present study.
This study is not without limitations. We employed a feasibility design with an insufficient sample size, thus limiting the applicability of the findings. Hence, studies with a more robust design and a larger sample size are recommended. Although the study primarily concentrated on the upper extremity of the post-stroke patient, several other areas of impairment could benefit from TR, such as deficits in the lower limb. The majority of the participants are middle-aged males with ischemic stroke; thus, the findings of the study should be interpreted with caution. Hence, future studies with representative samples are warranted. The TR mode of intervention would indisputably reduce clinical visits and waiting time. On the other hand, we believe TR may not be appropriate for individuals with severe impairments due to potential hindrances caused by worsened disabilities, such as spasticity and contracture, that may negatively affect motor skills [53].
However, the study has some strengths, as follows. The intervention facilitated two-way communication between physiotherapists and patients, thus providing a means for proper communication. The assessors were blinded, and the participants were randomly assigned to minimize bias and improve the study's validity. The audio-based TR contributes to a notable enhancement in functioning during the early stage of recovery. The TR recipients found it acceptable, completing almost 100% of therapy sessions with no reports of serious adverse events related to treatment.
Conclusion
This study revealed that TR intervention is feasible and shows significant promise in enhancing upper limb motor function and QOL among adult stroke survivors. Therefore, TR can be considered a complementary intervention to the conventional in-person approach to upper extremity rehabilitation among stroke patients and promote access and affordability of rehabilitation services. Further, this study suggests that using TR to deliver upper limb rehabilitation could be superior to conventional methods in improving motor function and QOL among stroke patients. However, further studies in the form of randomized controlled trials with larger representative samples are warranted to validate the effectiveness of the TR intervention.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of the Ministry of Health and Human Services (Code: MOH/GEN/747/001/01) granted the study's approval. The study was registered at Pan African Clinical Trials (Code: PACTR202301605291913).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and Methodology: Hafsat Maina Ali, Surajo Kamilu Sulaiman, and Umar Muhammad Bello; Supervision and data analysis: Surajo Kamilu Sulaiman and Umar Muhammad Bello; Data collection: Abdullahi Salisu Muhammad, Fatima Mahmud Ado, Hassan Ali Maina, Hussaina Abubakar Jalo, Mohammed Usman Ali, Ismail Muhammad Bello, and Halima Maina; Writing the original draft: Hafsat Maina Ali, Abdullahi Salisu Muhammad, Fatima Mahmud Ado, Hassan Ali Maina, Hussaina Abubakar Jalo, Mohammed Usman Ali, Ismail Muhammad Bello, and Halima Maina; Review and editing: Surajo Kamilu Sulaiman and Umar Muhammad Bello.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors are grateful to Fatima Sa’ad for contributing to the recruitment of the participants and Stephen Chinonso Ogbunamiri for providing technical support.
References
- Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. “Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001; 32(6):1279-84. [DOI:10.1161/01.str.32.6.1279] [PMID]
- Baron JC. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nature Reviews. Neurology. 2018; 14(6):325-37. [DOI:10.1038/s41582-018-0002-2] [PMID]
- Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: Estimates from monitoring, surveillance, and modelling. The Lancet. Neurology. 2009; 8(4):345-54. [DOI:10.1016/S1474-4422(09)70023-7] [PMID]
- Wittenauer BR, Smith L. Priority medicines for Europe and the world " a public health approach to innovation " update on 2004 background paper written by Eduardo Sabaté and Sunil Wimalaratna background paper 6. Ischaemic and haemorrhagic. Geneva: World Health Organisation; 2012.
- Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. The New England Journal of Medicine. 2010; 362(19):1772-83. [DOI:10.1056/NEJMoa0911341] [PMID]
- Yavuzer G, Selles R, Sezer N, Sütbeyaz S, Bussmann JB, Köseoğlu F, et al. Mirror therapy improves hand function in subacute stroke: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation. 2008; 89(3):393-8. [DOI:10.1016/j.apmr.2007.08.162] [PMID]
- Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016; 47(6):e98-169. [PMID]
- Roby-Brami A, Jarrassé N, Parry R.“Impairment and compensation in dexterous upper-limb function after stroke. From the direct consequences of pyramidal tract lesions to behavioral involvement of both upper-limbs in daily activities. Frontiers in Human Neuroscience. 2021; 15:662006. [DOI:10.3389/fnhum.2021.662006] [PMID]
- Rogante M, Grigioni M, Cordella D, Giacomozzi C. Ten years of telerehabilitation: A literature overview of technologies and clinical applications. NeuroRehabilitation. 2010; 27(4):287-304. [DOI:10.3233/NRE-2010-0612] [PMID]
- Clarke DJ, Forster A. Improving post-stroke recovery: The role of the multidisciplinary health care team. Journal of Multidisciplinary Healthcare. 2015; 8:433-42. [DOI:10.2147/JMDH.S68764] [PMID]
- McCue M, Fairman A, Pramuka M. Enhancing quality of life through telerehabilitation. Physical Medicine and Rehabilitation Clinics of North America. 2010; 21(1):195-205. [DOI:10.1016/j.pmr.2009.07.005] [PMID]
- Borschmann KN, Hayward KS. Recovery of upper limb function is greatest early after stroke but does continue to improve during the chronic phase: A two-year, observational study. Physiotherapy. 2020; 107:216-23. [DOI:10.1016/j.physio.2019.10.001] [PMID]
- Shaukat N, Ali DM, Razzak J. Physical and mental health impacts of COVID-19 on healthcare workers: A scoping review. International Journal of Emergency Medicine. 2020; 13(1):40. [DOI:10.1186/s12245-020-00299-5] [PMID]
- Calvaresi D, Schumacher M, Marinoni M, Hilfiker R, Dragoni AF, Buttazzo G. Agent-based systems for telerehabilitation: strengths, limitations and future challenges. In: Montagna S, Abreu P, Giroux S, Schumacher M, editors. Agents and multi-agent systems for health care. A2HC AHEALTH 2017 2017. Lecture Notes in Computer Science(), vol 10685. [DOI:10.1007/978-3-319-70887-4_1]
- Sarfo FS, Ulasavets U, Opare-Sem OK, Ovbiagele B. “Tele-Rehabilitation after Stroke: An updated systematic review of the literature. Journal of Stroke and Cerebrovascular Diseas 2018; 27(9):2306-18. [DOI:10.1016/j.jstrokecerebrovasdis.2018.05.013]
- Saito T, Izawa KP. Effectiveness and feasibility of home-based telerehabilitation for community-dwelling elderly people in Southeast Asian countries and regions: A systematic review. Aging Clinical and Experimental Research. 2021; 33:2657–69. [DOI:10.1007/s40520-021-01820-3]
- Chen J, Liu M, Sun D, Jin Y, Wang T, Ren C. Effectiveness and neural mechanisms of home-based telerehabilitation in patients with stroke based on fMRI and DTI: A study protocol for a randomized controlled trial. Medicine (Baltimore). 2018; 97(3):e9605. [PMID]
- Appleby E, Gill ST, Hayes LK, Walker TL, Walsh M, Kumar S. Effectiveness of telerehabilitation in the management of adults with stroke : A systematic review. PLoS One. 2019; 14(11):e0225150. [DOI:10.1371/journal.pone.0225150] [PMID]
- Langan J, Delave K, Phillips L, Pangilinan P, Brown SH. Home-based telerehabilitation shows improved upper limb function in adults with chronic stroke : A pilot study. Journal of Rehabilitation Medicine. 2013; 45(2):217–20. [DOI:10.2340/16501977-1115]
- Adhikari SP, Shrestha P, Dev R. Feasibility and effectiveness of telephone-based telephysiotherapy for treatment of pain in low-resource setting: A retrospective pre-post design. Pain Research & Management. 2020; 2020:2741278. [PMID]
- Øra HP, Kirmess M, Brady MC, Sørli H, Becker F. Technical features, feasibility, and acceptability of augmented telerehabilitation in post-stroke aphasia-experiences from a randomized controlled Trial. Front Neurol. 2020; 11:671. [DOI: 10.3389/fneur.2020.00671] [PMID]
- Chen J, Sun D, Zhang S, Shi Y, Qiao F, Zhou Y, et al. Effects of home-based telerehabilitation in patients with stroke: A randomized controlled trial. Neurology. 2020; 95(17):e2318-30. [DOI:10.1212/WNL.0000000000010821] [PMID]
- Kessler D, Anderson ND, Dawson DR. Occupational performance coaching for stroke survivors delivered via telerehabilitation using a single-case experimental design. The British Journal of Occupational Therapy. 2021; 84(8):488-96. [DOI:10.1177/0308022620988471] [PMID]
- Leochico CFD, Austria EMV, Gelisanga MAP, Ignacio SD, Mojica JAP. Home-based telerehabilitation for community-dwelling persons with stroke during the covid-19 pandemic: A pilot study. Journal of Rehabilitation Medicine. 2023; 55:jrm4405. [PMID]
- Alonazi A. Effectiveness and acceptability of telerehabilitation in physical therapy during covid-19 in children: Findings of a systematic review. Children (Basel). 2021; 8(12):1101. [DOI:10.3390/children8121101] [PMID]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013; 310(20):2191–4. [DOI: 10.1001/jama.2013.281053] [PMID]
- Rohrich RJ. Ethical approval of clinical studies, informed consent, and the declaration of Helsinki: What you need to know. Plastic and Reconstructive Surgery. 2007; 119(7):2307-9. [DOI:10.1097/01.prs.0000229193.31894.d0] [PMID]
- Coen RF, McCarroll K, Casey M, McNulty H, Laird E, Molloy AM, et al. The frontal assessment battery: Normative performance in a large sample of older community-dwelling hospital outpatient or general practitioner attenders. Journal of Geriatric Psychiatry and Neurology. 2016; 29(6):338-43. [DOI:10.1177/0891988716666381] [PMID]
- Mohan U, Babu SK, Kumar KV, Suresh BV, Misri ZK, Chakrapani M. Effectiveness of mirror therapy on lower extremity motor recovery, balance and mobility in patients with acute stroke: A randomized sham-controlled pilot trial. Annals of Indian Academy of Neurology. 2013; 16(4):634-9. [DOI:10.4103/0972-2327.120496] [PMID]
- Dettmers C, Nedelko V, Hassa T, Starrost K, Schoenfeld MA. Video Therapy’: Promoting hand function after stroke by action observation training - a pilot randomized controlled trial. International Journal of Physical Medicine & Rehabilitation. 2014; 2(189):1-7. [Link]
- Dodakian L, McKenzie AL, Le V, See J, Pearson-Fuhrhop K, Burke Quinlan E, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabilitation and Neural Repair. 2017; 31(10-11):923-33. [DOI:10.1177/1545968317733818] [PMID]
- Lin JH, Hsueh IP, Sheu CF, Hsieh CL. Psychometric properties of the sensory scale of the Fugl-Meyer Assessment in stroke patients. Clinical Rehabilitation. 2004; 18(4):391-7. [DOI:10.1191/0269215504cr737oa] [PMID]
- Hiragami S, Inoue Y, Harada K. Minimal clinically important difference for the Fugl-Meyer assessment of the upper extremity in convalescent stroke patients with moderate to severe hemiparesis. Journal of Physical Therapy Science. 2019; 31(11):917-21. [DOI:10.1589/jpts.31.917] [PMID]
- Wolf SL, Thompson PA, Morris DM. The EXCITE trial: Attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabilitation and Neural Repair. 2005; 19(3):194-205. [DOI:10.1177/1545968305276663]
- Fritz SL, Blanton S, Uswatte G, Taub E, Wolf SL.“Minimal detectable change scores for the wolf motor function test. Neurorehabilitation and Neural Repair. 2009; 23(7):662-7. [DOI:10.1177/1545968309335975] [PMID]
- Wong FY, Yang L, Yuen JWM, Chang KKP, Wong FKY.Assessing quality of life using WHOQOL- BREF : A cross-sectional study on the association between quality of life and neighborhood environmental satisfaction, and the mediating effect of health-related behaviors. BMC Public Health. 2018; 18(1):1113. [DOI:10.1186/s12889-018-5942-3] [PMID]
- Power M, Quinn K, Schmidt S; WHOQOL-OLD Group.Development of the WHOQOL-Old module. Quality of life research. 2005; 14(10):2197-14. [DOI:10.1007/s11136-005-7380-9] [PMID]
- Trompenaars FJ, Masthoff ED, Van Heck GL, Hodiamont PP, De Vries J. Content validity, construct validity, and reliability of the WHOQOL-Bref in a population of Dutch adult psychiatric outpatients. Quality of Life Research. 2005; 14(1):151-60. [DOI:10.1007/s11136-004-0787-x] [PMID]
- Yeo SM, Lim JY, Do JG, Lim JY, In Lee J, Hwang JH. Effectiveness of interactive augmented reality-based telerehabilitation in patients with adhesive capsulitis: Protocol for a multi-center randomized controlled trial. BMC Musculoskeletal Disorders. 2021; 22(1):386. [DOI:10.1186/s12891-021-04261-1] [PMID]
- Division of mental health and prevention of substance abuse, WHO. WHOQOL measuring quality of life. Geneva: World Health Organization; 1997. [Link]
- Mahoney FI. Barthel DW. Functional evaluation: The Barthel index. MD State Med J. 1965; 14, 61-5. [PMID]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nature Reviews. Neurology. 2011; 7(2):76-85. [DOI:10.1038/nrneurol.2010.200] [PMID]
- Wanner M, Schönherr G, Kiechl S, Knoflach M, Müller C, Seebacher B. Feasibility of an individualised, task- supported home exercise programme for arm function in patients in the subacute phase after stroke : Protocol of a randomised controlled pilot study. BMJ Open. 2022; 12(1):e051504. [DOI:10.1136/bmjopen-2021-051504] [PMID]
- Piron L, Tonin P, Trivello E, Battistin L, Dam M. Motor tele-rehabilitation in post-stroke patients. Medical Informatics and the Internet in Medicine. 2004; 29(2):119-25. [DOI:10.1080/14639230410001723428] [PMID]
- Schulz KF, Altman DG, Moher D; CONSORT Group.“CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010; 340:c332. [DOI:10.1136/bmj.c332] [PMID]
- Denny MC, Vahidy F, Vu KY, Sharrief AZ, Savitz SI. Video-based educational intervention associated with improved stroke literacy, self- efficacy, and patient satisfaction. PLoS One. 2017; 12(3):e0171952. [DOI:10.1371/journal.pone.0171952] [PMID]
- Saywell NL, Vandal AC, Mudge S, Hale L, Brown P, Feigin V, et al. Telerehabilitation after stroke using readily available technology : A randomized controlled trial. Neurorehabilitation and Neural Repair. 2021; 35(1):88-97. [DOI:10.1177/1545968320971765] [PMID]
- Cramer SC, Dodakian L, Le V, See J, Augsburger R, McKenzie A, Zhou RJ, et al. “Efficacy of home-based telerehabilitation vs in-clinic therapy for adults after stroke a randomized clinical trial. JAMA Neurology. 2019; 76(9):1079-87. [DOI:10.1001/jamaneurol.2019.1604] [PMID]
- Contrada M, Arcuri F, Tonin P, Pignolo L, Mazza T, Nudo G, et al. Stroke Telerehabilitation in Calabria: A health technology assessment. Frontiers in Neurology.2022; 12:777608.[DOI:10.3389/fneur.2021.777608] [PMID]
- Chumbler NR, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients: A randomized, controlled trial. Stroke. 2012; 43(8):2168-74. [DOI:10.1161/STROKEAHA.111.646943] [PMID]
- Forducey PG, Glueckauf RL, Bergquist TF, Maheu MM, Yutsis M.Telehealth for persons with severe functional disabilities and their caregivers: Facilitating self-care management in the home setting. Psychological Services. 2012; 9(2):144-62. [DOI:10.1037/a0028112] [PMID]
- Laver KE, Adey-Wakeling Z, Crotty M, Lannin NA, George S, Sherrington C. Telerehabilitation services for stroke. The Cochrane Database of Systematic Reviews. 2020; 1(1):CD010255. [DOI:10.1002/14651858.CD010255.pub3] [PMID]
- Hüzmeli ED, Duman T, Yıldırım H. Efficacy of telerehabilitation in patients with stroke in Turkey: A pilot study. Turkish Journal of Neurology. 2017; 23(1):021-5. [DOI: 10.4274/tnd.37268]
Article type: Original Research Articles |
Subject:
Rehabilitation Management
Received: 2023/09/21 | Accepted: 2024/01/28 | Published: 2024/12/20
Received: 2023/09/21 | Accepted: 2024/01/28 | Published: 2024/12/20
Send email to the article author