Volume 23, Issue 3 (September 2025)
Iranian Rehabilitation Journal 2025, 23(3): 321-330 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Adeniji T, Nadasan T, Olagbegi O, Olumide D. Prevalence of Motor and Non-motor Impairments in Nigerian Stroke Survivors. Iranian Rehabilitation Journal 2025; 23 (3) :321-330
URL: http://irj.uswr.ac.ir/article-1-2114-en.html
URL: http://irj.uswr.ac.ir/article-1-2114-en.html
1- Discipline of Physiotherapy, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa.
2- Department of Physiotherapy, College of Medicine, University of Ibadan, Ibadan, Nigeria.
2- Department of Physiotherapy, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Full-Text [PDF 538 kb]
(392 Downloads)
| Abstract (HTML) (3304 Views)
Full-Text: (183 Views)
Introduction
Stroke is a major global health concern, causing significant disability and loss of function. Risk factors, such as hypertension and diabetes are closely associated with stroke mortality, accounting for a substantial proportion of stroke-related deaths [1]. Additionally, vascular dementia is common among stroke survivors, particularly in the older adult population, owing to the effects of aging [2]. Post-stroke symptoms that significantly affect the quality of life (QoL) of survivors include motor dysfunctions, such as hemiplegia, spasticity, and movement disorders, as well as non-motor symptoms, such as pain, cognitive impairment, balance dysfunction, speech impairment, and depression [3-5].
Studies have reported varying prevalence rates for these post-stroke symptoms, including factors such as stroke stage (acute to chronic), recruitment settings (community, acute care, neurology outpatient clinics), and specific research methodologies [3-7]. These disparities in findings underline the complexity of post-stroke symptoms and the need for more comprehensive studies.
In Nigeria, research on the prevalence of balance dysfunction, pain, and mild cognitive impairment among stroke survivors is limited. Vincent-Onabajo et al. [5] reported a prevalence of 36.8% balance impairment among stroke survivors undergoing neurorehabilitation. However, specific studies on the prevalence of pain and cognitive impairment in stroke survivors in Nigeria are lacking. It is worth noting that these symptoms are common among stroke survivors worldwide, with pain affecting almost 40% of survivors five years post-stroke [8].
Both preventable and unavoidable variables increase the risk of stroke. Non-modifiable risk factors include age, sex, family history, and race or ethnicity, whereas modifiable factors include high blood pressure, smoking, diet, and physical inactivity [4, 9]. In a study conducted in Ethiopia, hypertension was identified as the most common risk factor for stroke, followed by family history, alcohol intake, smoking, and heart failure [9]. Understanding the impact of stroke symptoms and their prevalence is crucial, given their long-term consequences and debilitating effects on survivors. This study was thus designed to investigate the prevalence of pain, motor, and cognitive impairments in older adult stroke survivors at the Osun State University Teaching Hospital in Osogbo City, Nigeria. By conducting this study, valuable insights can be gained into the prevalence and impact of these symptoms, which can inform the development of effective interventions for the care of older adults with stroke. This study will contribute to reducing the burden of stroke and improving the QoL of stroke survivors in this region.
Materials and Methods
A cross-sectional design was employed for this investigation, conducted at Osun State University Teaching Hospital, Osogbo City, Nigeria, and was part of a protocol published in the Brain Hemorrhage Journal. [10] The study’s participants consisted of stroke patients receiving care at the hospital’s physiotherapy department, and the inclusion criteria were patients aged 55 years or older with a confirmed medical diagnosis of stroke by neurologists who provided informed consent. Participants with visual impairments that could limit their participation in the assessments were excluded, as were those with a medical diagnosis of mental disorders by psychiatric or mental health experts.
Assessment of pain, motor, and cognitive dysfunction
Pain assessment: Pain levels were evaluated using the face pain scale-revised (FPSR) and the numerical rating scale (NRS), a vertical numerical pain rating scale accompanied by facial expressions. Patients rated their pain intensity by indicating the number or facial expression on the scale that best described their pain, using a range of values from 0 (indicating no pain) to 10 (representing the most intense pain). This scale is recognized for its reliability and validity in measuring post-stroke pain [11].
Motor dysfunction and balance assessment: Motor dysfunction and balance were assessed using the time up and go (TUG) test. This test measured the time taken by the patients to rise from a chair, walk 3 m at a safe and comfortable pace, turn, return to the chair, and sit down. The time taken to complete the task was recorded in seconds. The TUG test is recognized for its reliability and validity in distinguishing between patients with chronic stroke and healthy elderly participants, as well as for its correlation with lower limb impairments and locomotor capacities [12].
Cognitive assessment: The cognitive status of the patients was assessed using the mini-mental state examination (MMSE), a brief quantitative assessment of cognitive function. It comprises 11 questions covering aspects, such as orientation, memory, attention, language, and visual construction. The overall score ranges from 0 to 30, with lower scores indicating greater cognitive impairment. The scale is recognized for its reliability and validity in screening cognitive impairment in older adults [13, 14].
Sample size
The sample size was determined using the Equation 1 [15, 16]:
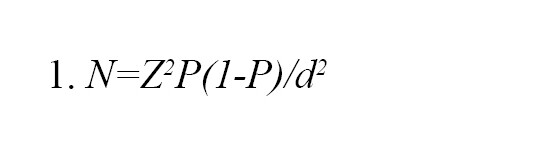
Where N represents the required sample size, Z is the z-score that corresponds to confidence level of 95%, equivalent to 1.96), P signifies the expected frequency (22% for cognitive impairment), and d represents precision (5%). Based on these values, the sample size was calculated to be 264.
Statistical analysis
Descriptive statistics were used to present the participants’ sociodemographic characteristics and comorbidities, including frequencies, percentages and Mean±SD. The prevalence of pain, motor, and cognitive dysfunction was calculated as a percentage. Respondents’ ages were categorized into three groups: Young-older adults (55-64 years), middle-aged adults (65-74 years), and oldest-older adults (>75 years).
A binary logistic regression model was utilized for inferential statistics to determine the sociodemographic and comorbid factors associated with the prevalence of stroke symptoms. The independent variables included age group, sex, ethnicity, side of the body affected by stroke, type of stroke, education level, tobacco use, alcohol consumption, employment status, high blood pressure, diabetes, TUG test results, and pain assessment (FPSR and NRS). A significance level of 0.05 was used for statistical analysis.
Results
A total of 261 stroke survivors participated in this study. They had a Mean±SD age of 68.42±7.72 years, ranging from 55 to 96. Most participants (89.3%) were of Yoruba origin, while a few were of Igbo or Hausa descent (5.0% and 5.7%, respectively).
The majority of the participants had secondary school education (52.1%). This was followed by participants with primary school education (38.3%) and tertiary (9.6%). Table 1 presents the sociodemographic and clinical characteristics of participants.
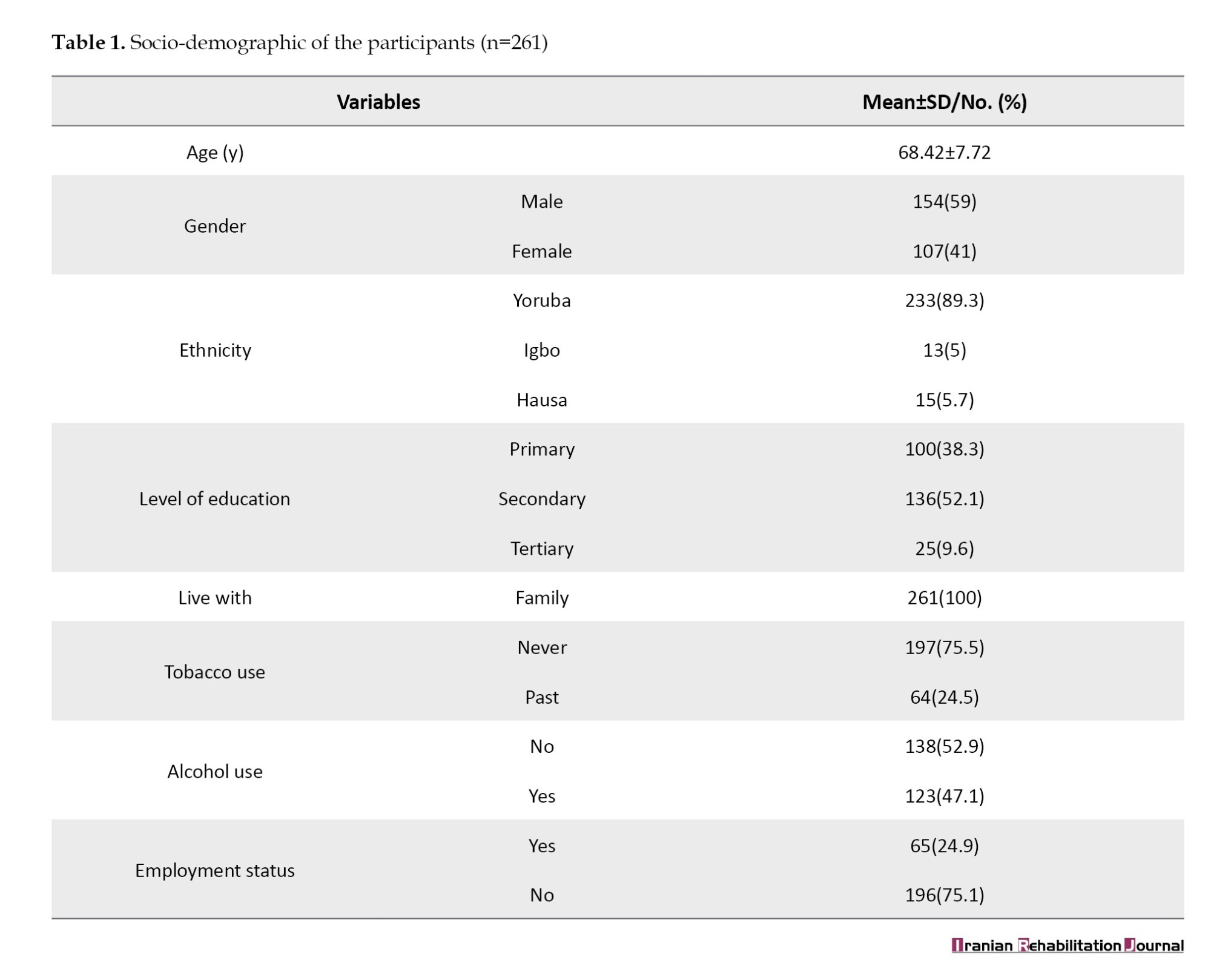
Table 2 presents the patients' clinical characteristics. The results showed that the majority of participants had ischemic stroke (62.5%). The participants' pain scores, as measured by the face and NRS pain scale, varied from 0 to 8, with a mean value of 3.72±2.66.
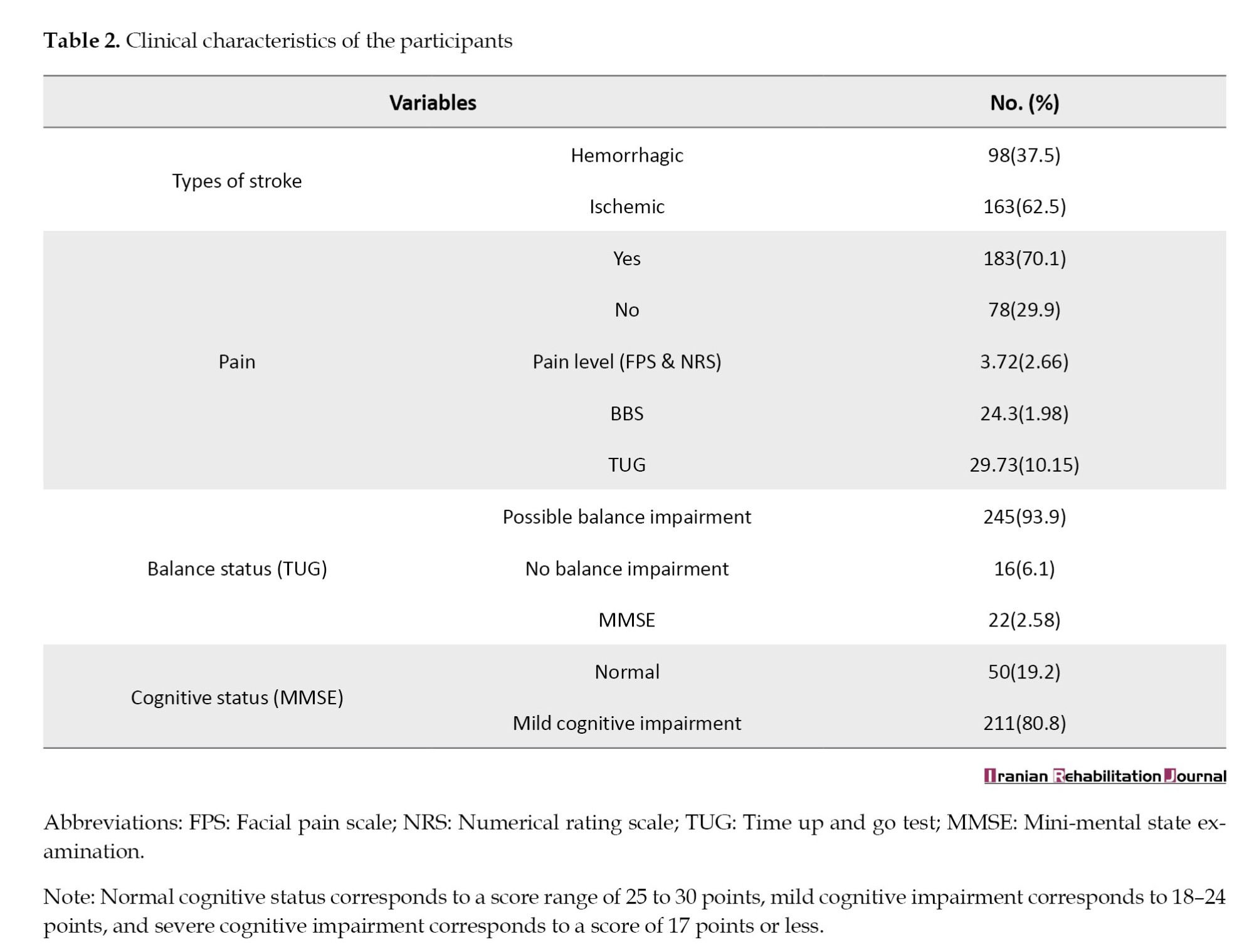
The prevalence of pain in this cohort of stroke survivors was 70.1%. The balance status of the participants, as measured by the TUG test, showed that 93.9% of the participants had balance impairment. Approximately 80.8% of participants had mild cognitive impairment.
Table 3 presents the prevalence of pain, motor and cognitive impairment symptoms among stroke survivors. The results showed that the age group 65-75 years had the highest prevalence of pain (41%), followed by those >75 years (33.3%), and finally by the age group- 55-64 years (25.6%). Male participants had higher pain prevalence (53.8%) than female participants (46.2%), and patients with ischemic stroke reported higher pain (62.3%) than stroke survivors with hemorrhagic stroke. The prevalence of pain was higher among participants with secondary school education (49.2%) than among those with primary (42.1%) and tertiary (8.7%) education.
In Table 3, the prevalence of mild cognitive impairment was highest in the age categories between 65 and 75 years (44.5%), followed by those between 55 and 64 years (32.2%), and finally by those in the age bracket of 75 years and above (23.2%). In gender categories, more male participants (55.9%) had mild cognitive impairment than female participants (44.1%). Ischemic stroke survivors (63.5%) had higher mild cognitive impairment compared to the participants with hemorrhagic stroke (36.5%). Mild cognitive impairment was higher among those with alcohol use (55.0%) and low education (secondary-50.2% and primary-39.3%). Table 3 shows that participants in the 65-75-year age group had the highest prevalence of balance impairment (42.9%), compared to the 55-64-year age group (33.9%) and those above 75 years (23.3%). Male participants had a higher prevalence of motor impairment (55.9%), and balance impairment was higher among participants with right-sided body affectation (58.8%).
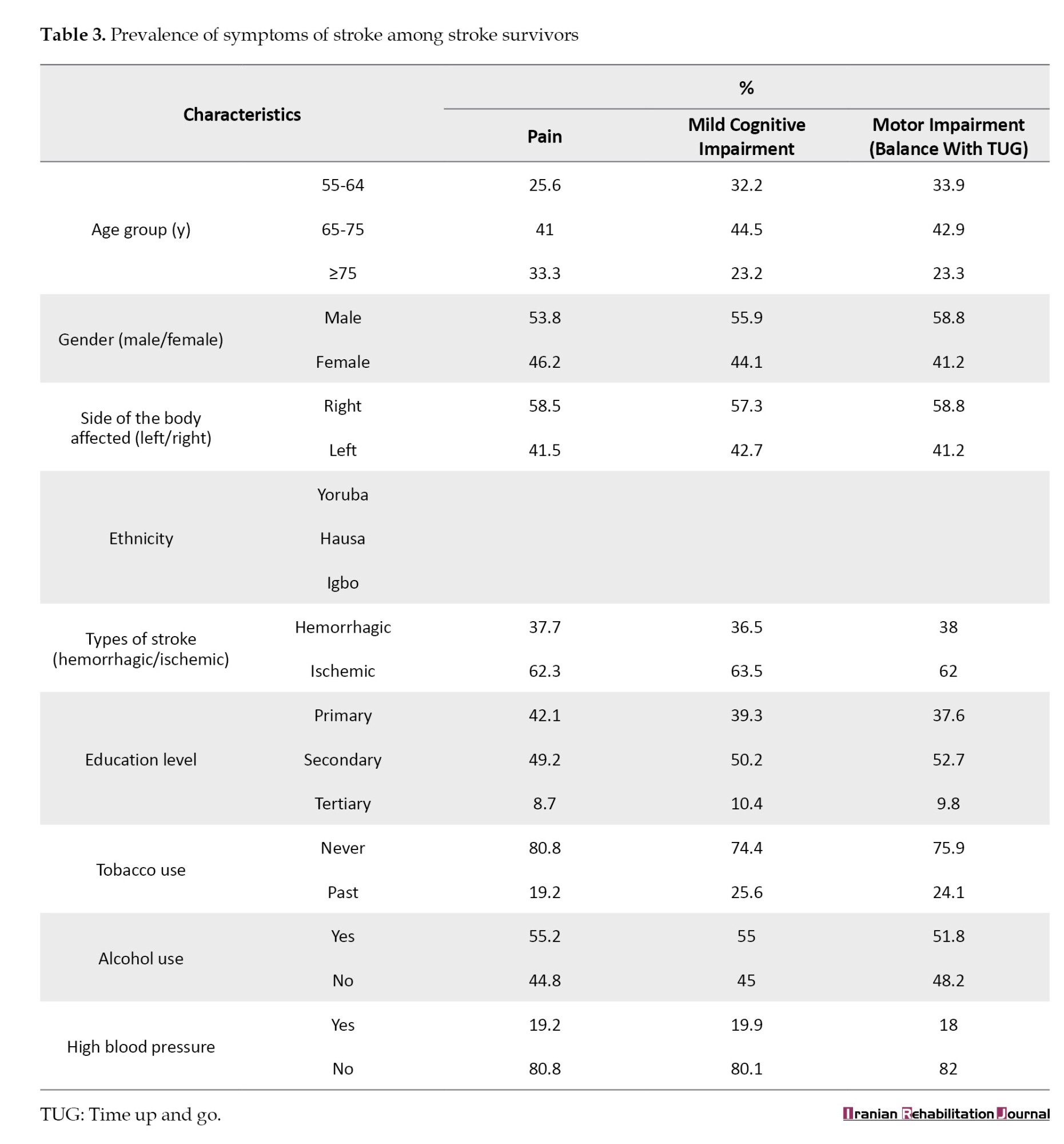
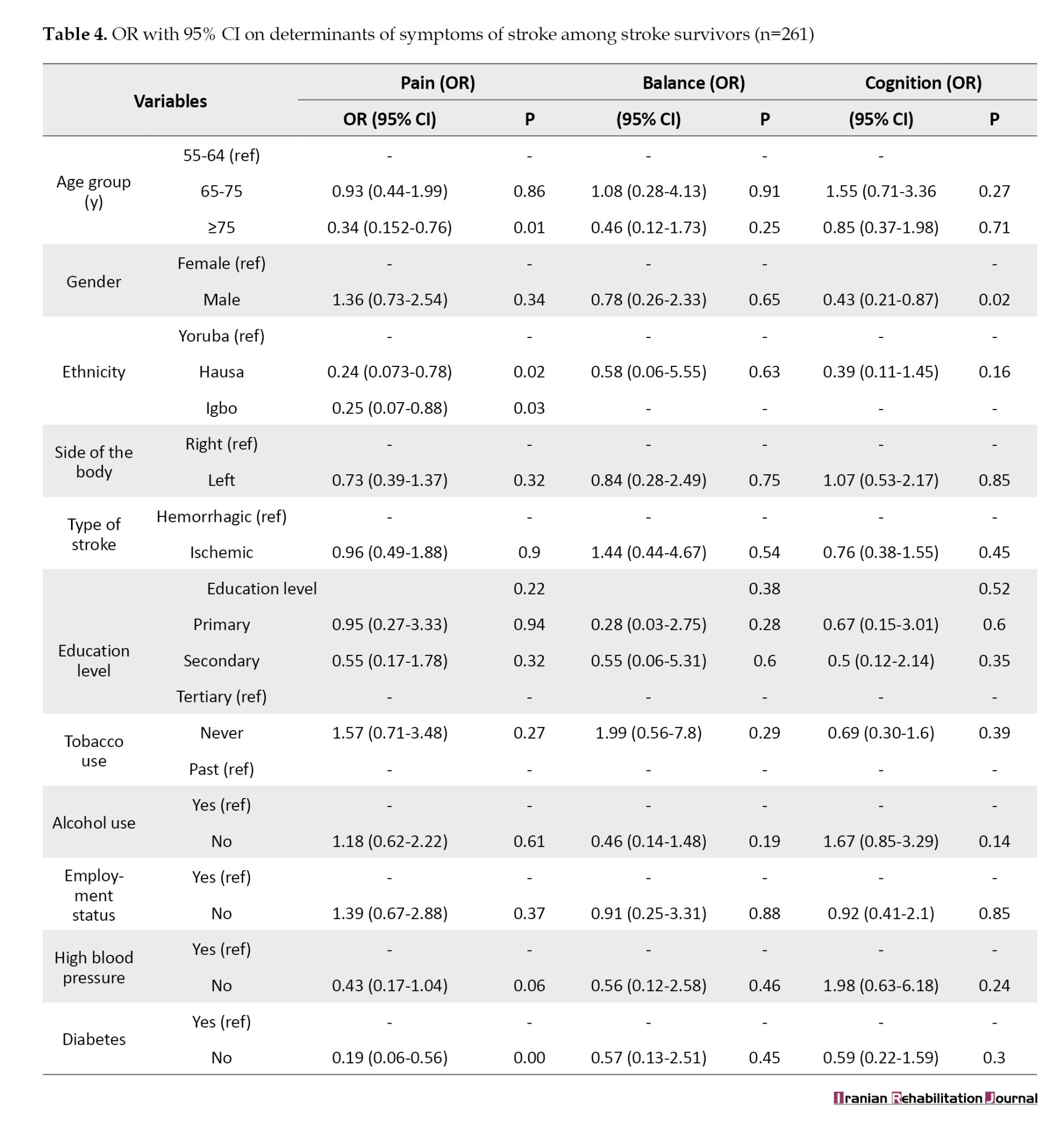
Table 4 presents the results of the odds ratio (OR) and determinants of stroke symptoms among stroke survivors. Participants who were 75 years old or older had a considerably lower likelihood of experiencing pain compared to those who were between the ages of 55 and 64 (OR=0.34, 95% CI, 0.15%, 0.78%). Hausa participants were significantly less likely to have pain compared to Yoruba population (OR=0.24, 95% CI, 0.07%, 0.78%). Similarly, Igbo participants were significantly less likely to have pain compared to the Yoruba population (OR=0.25, 95% CI, 0.07%, 0.88%). Participants without diabetes were significantly less likely to have pain compared to those who had diabetes (OR=0.19, 95% CI, 0.06%, 0.56%). Regarding cognitive impairment, male participants were significantly less likely to have mild cognitive impairment compared to females (OR=0.43, 95% CI, 0.21%, 0.87%). However, no significant correlations were found between balance impairment and the other variables.
Discussion
This study aimed to assess the prevalence of pain and motor and cognitive impairments in older adult patients with stroke and to investigate the relationships between these impairments and various sociodemographic and clinical variables. This study aimed to provide insights into the impact of stroke on survivors' QoL and inform the development of interventions to enhance their outcomes. This study was conducted in Osun State, Nigeria, and focused on a predominantly Yoruba ethnic group, reflecting the region's demographic composition. Furthermore, the study highlighted that most stroke survivors had low education levels, potentially affecting their access to healthcare, rehabilitation services, and social support [17].
The findings indicated a high prevalence of balance impairment, with 93.9% of participants exhibiting potential balance issues. Previous studies have reported varying prevalence rates of balance impairment, ranging from 36.8% to 83%, depending on demographic factors, such as age, sex, stroke type, alcohol use, and education level [5, 7, 18]. Older age groups (65-75 and >75 years) showed a higher prevalence of balance impairment, likely due to the greater impact of stroke on motor and sensory functions in the elderly, coupled with comorbidities, such as osteoporosis, arthritis, and vision problems [19, 20].
The type and location of the stroke also influence balance impairment, with ischemic strokes associated with a higher likelihood of balance issues due to their impact on cortical brain regions responsible for higher-order cognitive processes. In contrast, hemorrhagic strokes affect subcortical regions linked to motor and sensory functions [21, 22]. This study emphasized the need to tailor balance interventions according to specific deficits associated with the type and location of stroke.
Furthermore, stroke survivors who had never used tobacco were less likely to experience balance impairment, possibly due to the detrimental effects of tobacco on the vascular system. Tobacco use can impair the vascular supply and function of the brain and peripheral nerves, thereby affecting motor and sensory abilities [23, 24]. This study recommended screening for tobacco use in stroke survivors and providing smoking cessation programs for current smokers to prevent relapse among former smokers.
The study emphasized the importance of a holistic and personalized approach in evaluating and addressing balance issues in stroke survivors, considering various factors, such as age, sex, ethnicity, stroke side and type, and lifestyle factors such as tobacco and alcohol consumption, diabetes, and hypertension. This individualized approach can enhance the QoL and recovery of stroke survivors.
Regarding cognitive impairment, the study revealed a significant prevalence of mild cognitive impairment among stroke survivors. This finding highlights the challenges faced by stroke survivors regarding their cognitive functioning. Mild cognitive impairment was found to vary with factors, such as age, sex, stroke type, alcohol use, and educational level. Older age groups (65-75 and >75 years) had a higher prevalence of mild cognitive impairment, possibly due to the greater impact of stroke on cognitive function and the presence of comorbidities, such as vascular disease, diabetes, and hypertension [25, 26].
The study also showed that a substantial proportion of stroke survivors (70.1%) experienced pain primarily associated with ischemic stroke. Pain intensity varied among participants, with a moderate mean score. The prevalence of pain observed in this study was higher than that reported in previous studies, emphasizing the impact of different pain types and post-stroke stages. [8, 27, 28] Pain after stroke reduced QoL, hindered daily activities and occupation, and increased depressive disorders.
Older age groups (>75 years) exhibited a lower likelihood of experiencing pain than younger age group (55-64 years). This observation could be attributed to older stroke survivors having increased pain tolerance and better coping strategies as well as easier access to pain relief medications [29, 30]. This study emphasized the need to assess pain intensity in older stroke survivors and not to underestimate their pain based on age.
This study also found that stroke survivors with ischemic stroke were less likely to experience pain than those with hemorrhagic stroke, reflecting the different mechanisms and locations of brain damage associated with each stroke type. Ischemic strokes are more likely to affect the thalamus, which plays a critical role in pain signal processing and modulation, potentially resulting in central post-stroke pain (CPSP). In contrast, hemorrhagic stroke is more likely to affect subcortical brain regions, leading to peripheral post-stroke pain (PPSP), which is often acute and responsive to anti-inflammatory drugs [31–33]. This study recommends screening for CPSP and PPSP in stroke survivors and providing tailored treatments targeting the underlying neural mechanisms of these pain types.
Conclusion
The findings of this study highlight the need for a comprehensive and individualized approach to assessing and managing pain, mild cognitive impairment, and balance impairment in stroke survivors, considering their age, sex, stroke type, alcohol use, education level, and other relevant factors. By understanding the factors that influence pain, mild cognitive impairment, and balance impairment after stroke, healthcare professionals can better tailor their interventions to the specific needs and preferences of each stroke survivor and improve their QoL and recovery.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Osun State University Teaching Hospital, Osogbo, Nigeria (Code: UTH/REC/2022/08/602) and University of KwaZulu-Natal, Durban, South Africa (Code: BREC/00004363/2022).
Funding
This research was financially supported by the University of KwaZulu-Natal, Durban, South Africa.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the support of the Osun State University Teaching Hospital patients for their participation and the staff for the opportunity to collect data.
References
Stroke is a major global health concern, causing significant disability and loss of function. Risk factors, such as hypertension and diabetes are closely associated with stroke mortality, accounting for a substantial proportion of stroke-related deaths [1]. Additionally, vascular dementia is common among stroke survivors, particularly in the older adult population, owing to the effects of aging [2]. Post-stroke symptoms that significantly affect the quality of life (QoL) of survivors include motor dysfunctions, such as hemiplegia, spasticity, and movement disorders, as well as non-motor symptoms, such as pain, cognitive impairment, balance dysfunction, speech impairment, and depression [3-5].
Studies have reported varying prevalence rates for these post-stroke symptoms, including factors such as stroke stage (acute to chronic), recruitment settings (community, acute care, neurology outpatient clinics), and specific research methodologies [3-7]. These disparities in findings underline the complexity of post-stroke symptoms and the need for more comprehensive studies.
In Nigeria, research on the prevalence of balance dysfunction, pain, and mild cognitive impairment among stroke survivors is limited. Vincent-Onabajo et al. [5] reported a prevalence of 36.8% balance impairment among stroke survivors undergoing neurorehabilitation. However, specific studies on the prevalence of pain and cognitive impairment in stroke survivors in Nigeria are lacking. It is worth noting that these symptoms are common among stroke survivors worldwide, with pain affecting almost 40% of survivors five years post-stroke [8].
Both preventable and unavoidable variables increase the risk of stroke. Non-modifiable risk factors include age, sex, family history, and race or ethnicity, whereas modifiable factors include high blood pressure, smoking, diet, and physical inactivity [4, 9]. In a study conducted in Ethiopia, hypertension was identified as the most common risk factor for stroke, followed by family history, alcohol intake, smoking, and heart failure [9]. Understanding the impact of stroke symptoms and their prevalence is crucial, given their long-term consequences and debilitating effects on survivors. This study was thus designed to investigate the prevalence of pain, motor, and cognitive impairments in older adult stroke survivors at the Osun State University Teaching Hospital in Osogbo City, Nigeria. By conducting this study, valuable insights can be gained into the prevalence and impact of these symptoms, which can inform the development of effective interventions for the care of older adults with stroke. This study will contribute to reducing the burden of stroke and improving the QoL of stroke survivors in this region.
Materials and Methods
A cross-sectional design was employed for this investigation, conducted at Osun State University Teaching Hospital, Osogbo City, Nigeria, and was part of a protocol published in the Brain Hemorrhage Journal. [10] The study’s participants consisted of stroke patients receiving care at the hospital’s physiotherapy department, and the inclusion criteria were patients aged 55 years or older with a confirmed medical diagnosis of stroke by neurologists who provided informed consent. Participants with visual impairments that could limit their participation in the assessments were excluded, as were those with a medical diagnosis of mental disorders by psychiatric or mental health experts.
Assessment of pain, motor, and cognitive dysfunction
Pain assessment: Pain levels were evaluated using the face pain scale-revised (FPSR) and the numerical rating scale (NRS), a vertical numerical pain rating scale accompanied by facial expressions. Patients rated their pain intensity by indicating the number or facial expression on the scale that best described their pain, using a range of values from 0 (indicating no pain) to 10 (representing the most intense pain). This scale is recognized for its reliability and validity in measuring post-stroke pain [11].
Motor dysfunction and balance assessment: Motor dysfunction and balance were assessed using the time up and go (TUG) test. This test measured the time taken by the patients to rise from a chair, walk 3 m at a safe and comfortable pace, turn, return to the chair, and sit down. The time taken to complete the task was recorded in seconds. The TUG test is recognized for its reliability and validity in distinguishing between patients with chronic stroke and healthy elderly participants, as well as for its correlation with lower limb impairments and locomotor capacities [12].
Cognitive assessment: The cognitive status of the patients was assessed using the mini-mental state examination (MMSE), a brief quantitative assessment of cognitive function. It comprises 11 questions covering aspects, such as orientation, memory, attention, language, and visual construction. The overall score ranges from 0 to 30, with lower scores indicating greater cognitive impairment. The scale is recognized for its reliability and validity in screening cognitive impairment in older adults [13, 14].
Sample size
The sample size was determined using the Equation 1 [15, 16]:
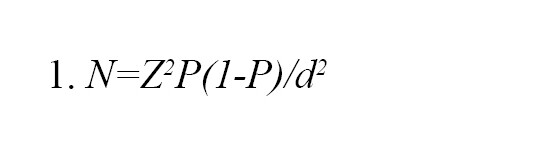
Where N represents the required sample size, Z is the z-score that corresponds to confidence level of 95%, equivalent to 1.96), P signifies the expected frequency (22% for cognitive impairment), and d represents precision (5%). Based on these values, the sample size was calculated to be 264.
Statistical analysis
Descriptive statistics were used to present the participants’ sociodemographic characteristics and comorbidities, including frequencies, percentages and Mean±SD. The prevalence of pain, motor, and cognitive dysfunction was calculated as a percentage. Respondents’ ages were categorized into three groups: Young-older adults (55-64 years), middle-aged adults (65-74 years), and oldest-older adults (>75 years).
A binary logistic regression model was utilized for inferential statistics to determine the sociodemographic and comorbid factors associated with the prevalence of stroke symptoms. The independent variables included age group, sex, ethnicity, side of the body affected by stroke, type of stroke, education level, tobacco use, alcohol consumption, employment status, high blood pressure, diabetes, TUG test results, and pain assessment (FPSR and NRS). A significance level of 0.05 was used for statistical analysis.
Results
A total of 261 stroke survivors participated in this study. They had a Mean±SD age of 68.42±7.72 years, ranging from 55 to 96. Most participants (89.3%) were of Yoruba origin, while a few were of Igbo or Hausa descent (5.0% and 5.7%, respectively).
The majority of the participants had secondary school education (52.1%). This was followed by participants with primary school education (38.3%) and tertiary (9.6%). Table 1 presents the sociodemographic and clinical characteristics of participants.

Table 2 presents the patients' clinical characteristics. The results showed that the majority of participants had ischemic stroke (62.5%). The participants' pain scores, as measured by the face and NRS pain scale, varied from 0 to 8, with a mean value of 3.72±2.66.

The prevalence of pain in this cohort of stroke survivors was 70.1%. The balance status of the participants, as measured by the TUG test, showed that 93.9% of the participants had balance impairment. Approximately 80.8% of participants had mild cognitive impairment.
Table 3 presents the prevalence of pain, motor and cognitive impairment symptoms among stroke survivors. The results showed that the age group 65-75 years had the highest prevalence of pain (41%), followed by those >75 years (33.3%), and finally by the age group- 55-64 years (25.6%). Male participants had higher pain prevalence (53.8%) than female participants (46.2%), and patients with ischemic stroke reported higher pain (62.3%) than stroke survivors with hemorrhagic stroke. The prevalence of pain was higher among participants with secondary school education (49.2%) than among those with primary (42.1%) and tertiary (8.7%) education.
In Table 3, the prevalence of mild cognitive impairment was highest in the age categories between 65 and 75 years (44.5%), followed by those between 55 and 64 years (32.2%), and finally by those in the age bracket of 75 years and above (23.2%). In gender categories, more male participants (55.9%) had mild cognitive impairment than female participants (44.1%). Ischemic stroke survivors (63.5%) had higher mild cognitive impairment compared to the participants with hemorrhagic stroke (36.5%). Mild cognitive impairment was higher among those with alcohol use (55.0%) and low education (secondary-50.2% and primary-39.3%). Table 3 shows that participants in the 65-75-year age group had the highest prevalence of balance impairment (42.9%), compared to the 55-64-year age group (33.9%) and those above 75 years (23.3%). Male participants had a higher prevalence of motor impairment (55.9%), and balance impairment was higher among participants with right-sided body affectation (58.8%).

Table 4 presents the results of the odds ratio (OR) and determinants of stroke symptoms among stroke survivors. Participants who were 75 years old or older had a considerably lower likelihood of experiencing pain compared to those who were between the ages of 55 and 64 (OR=0.34, 95% CI, 0.15%, 0.78%). Hausa participants were significantly less likely to have pain compared to Yoruba population (OR=0.24, 95% CI, 0.07%, 0.78%). Similarly, Igbo participants were significantly less likely to have pain compared to the Yoruba population (OR=0.25, 95% CI, 0.07%, 0.88%). Participants without diabetes were significantly less likely to have pain compared to those who had diabetes (OR=0.19, 95% CI, 0.06%, 0.56%). Regarding cognitive impairment, male participants were significantly less likely to have mild cognitive impairment compared to females (OR=0.43, 95% CI, 0.21%, 0.87%). However, no significant correlations were found between balance impairment and the other variables.

Discussion
This study aimed to assess the prevalence of pain and motor and cognitive impairments in older adult patients with stroke and to investigate the relationships between these impairments and various sociodemographic and clinical variables. This study aimed to provide insights into the impact of stroke on survivors' QoL and inform the development of interventions to enhance their outcomes. This study was conducted in Osun State, Nigeria, and focused on a predominantly Yoruba ethnic group, reflecting the region's demographic composition. Furthermore, the study highlighted that most stroke survivors had low education levels, potentially affecting their access to healthcare, rehabilitation services, and social support [17].
The findings indicated a high prevalence of balance impairment, with 93.9% of participants exhibiting potential balance issues. Previous studies have reported varying prevalence rates of balance impairment, ranging from 36.8% to 83%, depending on demographic factors, such as age, sex, stroke type, alcohol use, and education level [5, 7, 18]. Older age groups (65-75 and >75 years) showed a higher prevalence of balance impairment, likely due to the greater impact of stroke on motor and sensory functions in the elderly, coupled with comorbidities, such as osteoporosis, arthritis, and vision problems [19, 20].
The type and location of the stroke also influence balance impairment, with ischemic strokes associated with a higher likelihood of balance issues due to their impact on cortical brain regions responsible for higher-order cognitive processes. In contrast, hemorrhagic strokes affect subcortical regions linked to motor and sensory functions [21, 22]. This study emphasized the need to tailor balance interventions according to specific deficits associated with the type and location of stroke.
Furthermore, stroke survivors who had never used tobacco were less likely to experience balance impairment, possibly due to the detrimental effects of tobacco on the vascular system. Tobacco use can impair the vascular supply and function of the brain and peripheral nerves, thereby affecting motor and sensory abilities [23, 24]. This study recommended screening for tobacco use in stroke survivors and providing smoking cessation programs for current smokers to prevent relapse among former smokers.
The study emphasized the importance of a holistic and personalized approach in evaluating and addressing balance issues in stroke survivors, considering various factors, such as age, sex, ethnicity, stroke side and type, and lifestyle factors such as tobacco and alcohol consumption, diabetes, and hypertension. This individualized approach can enhance the QoL and recovery of stroke survivors.
Regarding cognitive impairment, the study revealed a significant prevalence of mild cognitive impairment among stroke survivors. This finding highlights the challenges faced by stroke survivors regarding their cognitive functioning. Mild cognitive impairment was found to vary with factors, such as age, sex, stroke type, alcohol use, and educational level. Older age groups (65-75 and >75 years) had a higher prevalence of mild cognitive impairment, possibly due to the greater impact of stroke on cognitive function and the presence of comorbidities, such as vascular disease, diabetes, and hypertension [25, 26].
The study also showed that a substantial proportion of stroke survivors (70.1%) experienced pain primarily associated with ischemic stroke. Pain intensity varied among participants, with a moderate mean score. The prevalence of pain observed in this study was higher than that reported in previous studies, emphasizing the impact of different pain types and post-stroke stages. [8, 27, 28] Pain after stroke reduced QoL, hindered daily activities and occupation, and increased depressive disorders.
Older age groups (>75 years) exhibited a lower likelihood of experiencing pain than younger age group (55-64 years). This observation could be attributed to older stroke survivors having increased pain tolerance and better coping strategies as well as easier access to pain relief medications [29, 30]. This study emphasized the need to assess pain intensity in older stroke survivors and not to underestimate their pain based on age.
This study also found that stroke survivors with ischemic stroke were less likely to experience pain than those with hemorrhagic stroke, reflecting the different mechanisms and locations of brain damage associated with each stroke type. Ischemic strokes are more likely to affect the thalamus, which plays a critical role in pain signal processing and modulation, potentially resulting in central post-stroke pain (CPSP). In contrast, hemorrhagic stroke is more likely to affect subcortical brain regions, leading to peripheral post-stroke pain (PPSP), which is often acute and responsive to anti-inflammatory drugs [31–33]. This study recommends screening for CPSP and PPSP in stroke survivors and providing tailored treatments targeting the underlying neural mechanisms of these pain types.
Conclusion
The findings of this study highlight the need for a comprehensive and individualized approach to assessing and managing pain, mild cognitive impairment, and balance impairment in stroke survivors, considering their age, sex, stroke type, alcohol use, education level, and other relevant factors. By understanding the factors that influence pain, mild cognitive impairment, and balance impairment after stroke, healthcare professionals can better tailor their interventions to the specific needs and preferences of each stroke survivor and improve their QoL and recovery.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Osun State University Teaching Hospital, Osogbo, Nigeria (Code: UTH/REC/2022/08/602) and University of KwaZulu-Natal, Durban, South Africa (Code: BREC/00004363/2022).
Funding
This research was financially supported by the University of KwaZulu-Natal, Durban, South Africa.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors acknowledge the support of the Osun State University Teaching Hospital patients for their participation and the staff for the opportunity to collect data.
References
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392(10159):1859-922. [DOI:10.1016/S0140-6736(18)32335-3] [PMID]
- Di Carlo A, Baldereschi M, Amaducci L, Lepore V, Bracco L, Maggi S, et al. Incidence of dementia, Alzheimer's disease, and vascular dementia in Italy. The ILSA Study. Journal of the American Geriatrics Society. 2002; 50(1):41-8. [DOI:10.1046/j.1532-5415.2002.50006.x] [PMID]
- Clery A, Bhalla A, Rudd AG, Wolfe CDA, Wang Y. Trends in prevalence of acute stroke impairments: A population-based cohort study using the South London Stroke Register. PLOS Medicine. 2020; 17(10):e1003366. [DOI:10.1371/journal.pmed.1003366] [PMID]
- Zeng H, Chen J, Guo Y, Tan S. Prevalence and risk factors for spasticity after stroke: A systematic review and meta-analysis. Frontiers in Neurology. 2021; 11:616097.[DOI:10.3389/fneur.2020.616097] [PMID]
- Vincent-Onabajo G, Musa HY, Joseph E. Prevalence of balance impairment among stroke survivors undergoing neurorehabilitation in Nigeria. Journal of Stroke and cerebrovascular diseases. 2018; 27(12):3487-92 [DOI:10.1016/j.jstrokecerebrovasdis.2018.08.024] [PMID]
- Kuo CL, Hu GC. Post-stroke spasticity: A review of epidemiology, pathophysiology, and treatments. International Journal of Gerontology. 2018; 12(4):280-4. [DOI:10.1016/j.ijge.2018.05.005]
- Khan F, Chevidikunnan MF. Prevalence of balance impairment and factors associated with balance among patients with stroke. A cross sectional retrospective case control study. Healthcare (Basel). 2021; 9(3):320. [DOI:10.3390/healthcare9030320] [PMID]
- Westerlind E, Singh R, Persson HC, Sunnerhagen KS. Experienced pain after stroke: A cross-sectional 5-year follow-up study. BMC Neurology. 2020; 20(1):4 [DOI:10.1186/s12883-019-1584-z] [PMID]
- Fekadu G, Chelkeba L, Kebede A. Risk factors, clinical presentations and predictors of stroke among adult patients admitted to stroke unit of Jimma university medical center, south west Ethiopia: Prospective observational study. BMC Neurology. 2019; 19(1):187. [DOI:10.1186/s12883-019-1409-0] [PMID]
- Adeniji T, Nadasan T, Michael Olagbegi O, Dada O. Telerehabilitation-Based Exercises with or without Transcranial Direct Current Stimulation for Pain, Motor and Cognitive Function in Older Adults with mild Cognitive Impairments Post-Stroke: A Multi-Arm Parallel-Group Randomized Controlled Trial Study Protocol. Brain Hemorrhages. 2023; 4(3):122-8. [DOI:10.1016/j.hest.2023.01.004]
- Chuang LL, Wu CY, Lin KC, Hsieh CJ. Relative and absolute reliability of a vertical numerical pain rating scale supplemented with a faces pain scale after stroke. Physical Therapy. 2014; 94(1):129-38. [DOI:10.2522/ptj.20120422] [PMID]
- Ng SS, Hui-Chan CW. The timed up & go test: Its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Archives of Physical Medicine and Rehabilitation. 2005; 86(8):1641-7. [DOI:10.1016/j.apmr.2005.01.011]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12(3):189-98. [DOI:10.1016/0022-3956(75)90026-6] [PMID]
- Adeniji T, Oyeyemi AY. Translation, cultural adaptation and validation of the hausa version of the standardised mini-mental state examination in Northeastern Nigeria. West African Journal of Medicine. 2022;3 9(6):614-22. [PMID]
- Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London stroke register 1995–2010. Stroke. 2013; 44(1):138-45. [Link]
- Salari N, Lotfi F, Abdolmaleki A, Heidarian P, Rasoulpoor S, Fazeli J, Najafi H, Mohammadi M. The global prevalence of mild cognitive impairment in geriatric population with emphasis on influential factors: A systematic review and meta-analysis. BMC Geriatrics. 2025; 25(1):313. [Link]
- Lindmark A, Eriksson M, Darehed D. Socioeconomic status and stroke severity: Understanding indirect effects via risk factors and stroke prevention using innovative statistical methods for mediation analysis. Plos One. 2022; 17(6):e0270533. [DOI:10.1371/journal.pone.0270533] [PMID]
- Li J, Zhong D, Ye J, He M, Liu X, Zheng H, et al. Rehabilitation for balance impairment in patients after stroke: A protocol of a systematic review and network meta-analysis. BMJ Open. 2019; 9(7):e026844. [DOI:10.1136/bmjopen-2018-026844] [PMID]
- Falkenberg HK, Mathisen TS, Ormstad H, Eilertsen G. "Invisible" visual impairments. A qualitative study of stroke survivors` experience of vision symptoms, health services and impact of visual impairments. BMC Health Services Research. 2020; 20(1):302. [DOI:10.1186/s12913-020-05176-8] [PMID]
- Hreha K, Gupta P, Reistetter T. A case of comorbidities highlighting cerebral stroke, vision impairment, and dementia. SAGE Open Medical Case Reports. 2020; 2050313X20975246. [DOI:10.1177/2050313X20975246] [PMID]
- Liu Z, He S, Wei Y, Duan R, Zhang C, Li T, et al. Changes of cerebral cortical structure and cognitive dysfunction in "healthy hemisphere" after stroke: A study about cortical complexity and sulcus patterns in bilateral ischemic adult moyamoya disease. BMC Neuroscience. 2021; 22(1):66. [DOI:10.1186/s12868-021-00672-x] [PMID]
- McGurgan IJ, Ziai WC, Werring DJ, Al-Shahi Salman R, Parry-Jones AR. Acute intracerebral haemorrhage: Diagnosis and management. Practical Neurology. 2020; 21(2):128–36. [DOI:10.1136/practneurol-2020-002763] [PMID]
- Prahm C, Heinzel J, Kolbenschlag J. Blood Supply and Microcirculation of the Peripheral Nerve. In: Phillips JB, Hercher D, Hausner T, editors. Peripheral Nerve Tissue Engineering and Regeneration. Reference Series in Biomedical Engineering(). Cham: Springer; 2022. [DOI:10.1007/978-3-030-21052-6_21]
- Wang CH, Hsueh IP, Sheu CF, Yao G, Hsieh CL. Psychometric properties of 2 simplified 3-level balance scales used for patients with stroke. Physical Therapy. 2004; 84(5):430-8.[DOI:10.1093/ptj/84.5.430] [PMID]
- Morrison HW, White MM, Rothers JL, Taylor-Piliae RE. Examining the associations between post-stroke cognitive function and common comorbid conditions among stroke survivors. International Journal of Environmental Research and Public Health. 2022; 19(20):13445. [DOI:10.3390/ijerph192013445] [PMID]
- She J, Nakamura H, Makino K, Ohyama Y, Hashimoto H. Selection of suitable maximum-heart-rate formulas for use with Karvonen formula to calculate exercise intensity. International Journal of Automation and Computing. 2015; 12(1):62-9. [DOI:10.1007/s11633-014-0824-3]
- Paolucci S, Iosa M, Toni D, Barbanti P, Bovi P, Cavallini A, et al. Prevalence and time course of post-stroke pain: A multicenter prospective hospital-based study. Pain Medicine. 2016; 17(5):924-30. [DOI:10.1093/pm/pnv019] [PMID]
- Rufa’i AA, Oyeyemi AL, Kadafa AF, Lawan A, Saidu IA, Aliyu SU, et al. Musculoskeletal Pain after Stroke: Prevalence, patterns and distribution among survivors in Maiduguri, North Eastern Nigeria. Borno Medical Journal. 2019; 16(1):1-8. [Link]
- Cheng W, Tu J, Shen X. Registered nurses' role experiences of caring for older stroke patients: A qualitative study. BMC Nursing. 2021; 20(1):96. [DOI:10.1186/s12912-021-00626-y] [PMID]
- Kauffmann J, Grün D, Yilmaz U, Wagenpfeil G, Faßbender K, Fousse M, et al. Acute stroke treatment and outcome in the oldest old (90 years and older) at a tertiary care medical centre in Germany-a retrospective study showing safety and efficacy in this particular patient population. BMC Geriatrics. 2021; 21(1):611. [DOI:10.1186/s12877-021-02566-3] [PMID]
- Cao Z, Harvey SS, Bliss TM, Cheng MY, Steinberg GK. Inflammatory Responses in the Secondary Thalamic Injury After Cortical Ischemic Stroke. Frontiers in Neurology. 2020; 11:236.[DOI:10.3389/fneur.2020.00236] [PMID]
- Dierick F, Dehas M, Isambert JL, Injeyan S, Bouché AF, Bleyenheuft Y, et al. Hemorrhagic versus ischemic stroke: Who can best benefit from blended conventional physiotherapy with robotic-assisted gait therapy? PLoS One. 2017; 12(6):e0178636. [DOI:10.1371/journal.pone.0178636] [PMID]
- Hamzat TK, Osundiya OC. Musculoskeletal pain and its impact on motor performance among stroke survivors. Hong Kong Physiotherapy Journal. 2010; 28(1):11-5. [DOI:10.1016/j.hkpj.2010.11.001]
Article type: Original Research Articles |
Subject:
Neurorehabilitation
Received: 2023/10/22 | Accepted: 2024/04/7 | Published: 2025/09/1
Received: 2023/10/22 | Accepted: 2024/04/7 | Published: 2025/09/1
Send email to the article author








