Volume 15, Issue 3 (September 2017)
Iranian Rehabilitation Journal 2017, 15(3): 185-192 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Momeni F, Shiyasi Y, Mirzaasgari H. Psychometric Properties of the Persian Version of Brief Quality of Life Questionnaire in Bipolar Disorder. Iranian Rehabilitation Journal 2017; 15 (3) :185-192
URL: http://irj.uswr.ac.ir/article-1-705-en.html
URL: http://irj.uswr.ac.ir/article-1-705-en.html
1- Psychosis Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Department of Clinical Psychology, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Department of Clinical Psychology, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
Full-Text [PDF 541 kb]
(2508 Downloads)
| Abstract (HTML) (6708 Views)
Full-Text: (1431 Views)
1. Introduction
Bipolar Disorder (BD) is a common psychiatric disorder. It emerges as a syndrome with a complete set of symptoms of mania and depression and affects 1–2.4% of adults and 2.5% of adolescents [1]. Diagnostic and Statistical Model of Mental Disorders (DSM-V) defines BD as a period of mania and depression. The period of mania is characterized by abnormally elevated mood—open or irritable—that lasts for a week. During the period of openness, purposeful activity or level of energy is abnormally and constantly high, which is observable for most of the day and nearly all days. In addition, this period is characterized by such symptoms as increased self-confidence, grandiosity, flight of ideas; distraction, talkativeness, and decreased need for sleep [2].
BD is a chronic disorder with a high rate of relapse, and even when undergoing pharmacological treatment, about 40–60% of the patients have at least one relapse into depression or mania within 2 years. Patients with BD are 15–20 times more likely to commit suicide than that of the general population. In addition, more than 15% of the patients with BD have made at least one suicidal attempt in their lives [3].
Various studies have also shown that BD imposes a great deal of social costs on patients and people around them due to their decreased productivity. BD has a lower recovery rate and a higher mortality rate than that of other mood disorders. In 2000, the world health organization (WHO) ranked BD as the sixth leading cause of lifetime adjustment impairment in people in the age group of 15–44 years throughout the world [4]. The most troubling problem for these patients is suicidal impulses. Low Quality of Life (QoL) along with interpersonal conflicts has been observed in these patients during recovery [5]. QoL refers to each individual’s range of needs related to his/her perception about feeling good. Good QoL is not the absence of illness, but it refers to feeling good about various social, physical, and psychological functions [6].
QoL is a multidimensional and complex concept encompassing objective and subjective factors related to an individual’s perception of life satisfaction, physical and family health, hope, social interactions, and psychological health [7]. WHO defines QoL as an individual’s perception of their position in life and according to the cultural setting, value systems, and condition of the person’s life in relation to his/her goals, expectations, and standards [8]. There is evidence showing that the destructive effects of BD on the QoL of these patients is so severe that even in the normal mood phase, these patients are more vulnerable to having problems in their social and career lives compared to the patients with multiple sclerosis (MS) and rheumatoid arthritis [9].
Given that QoL is one of the most important constructs in assessing people’s vulnerability to physical and psychological impairments, various instruments have been designed to assess it, including the 36-Item Short-Form Health Survey (SF-36) questionnaire developed by the WHO; however, it seems that each questionnaire highlights a particular aspect of QoL, such as spirituality or attachment and ignoring the other aspects [10]. In addition, SF-36 is primarily used to assess the general QoL of individuals, and it is used for a wide range of people from healthy individuals to those with various physical and psychological disorders. However, there are not many instruments available to assess disorder-related QoL, including for BD.
The Quality of Life in Bipolar Disorder (QoL.BD) was the first instrument developed to assess BD-related QoL, and because it is disorder-specific, it appears to have a significant sensitivity and specificity relative to other QoL instruments. It has 56 items and 13 subscales. Because patients with BD may experience difficulty in answering a large number of items, the developers of the QoL.BD designed a short form of the questionnaire—the Brief Quality of Life in Bipolar Disorder (Brief QoL.BD). Brief QoL.BD assesses different aspects of the life of patients with BD, and the developers reported its good psychometric properties [11]. This questionnaire has been translated into some languages. The Spanish version showed a high reliability (α=0.95) and its convergent validity was relatively good (correlation coefficients with SF-36 ranging from 0.453 and 0.819; P<0.01). The Chinese version also had very high internal consistency (α=0.95) and retest reliability (interclass correlation coefficient about 0.808)
Due to the fact that assessing the QoL of patients with BD is both important for the diagnosis and examination of treatment outcomes, and given to the lack of Persian disorder-specific scale to assess QoL, the aim of this study was to consider psychometric properties of the Persian version of Brief QoL.BD in patients with BD in psychiatric hospitals of Tehran.
2. Methods
Population, sample, and sampling method
The statistical population comprised all the patients and normal persons in Tehran. The sample population included 118 patients suffering from BD and 110 healthy subjects. The sampling was an available one and the sample size was edited based on Gross Guideline which allocates 5–10 subjects to each question. The study participants were in two groups: the patient group and the healthy group. The patient group was suffering from BD being hospitalized in psychiatric hospitals for 1.5 months while had reached a relatively stable condition by taking mood stabilizer medications and showing signs of recovery. To confirm this, the patients who obtained a score of less than 13 in Beck Depression Inventory-II (BDI-II) and less than 6 in Beck–Rafaelsen Mania Rating Scale (BRMAS) were selected for further study.
The sampling was performed from a number of psychiatric hospitals in Tehran (Razi Psychiatric Center, Meymanat Psychiatric Hospital, Imam Hossein Hospital, Taleghani Hospital, Baghiatallah Hospital, and Azadi Psychiatric Center), and it lasted from March to December 2016. The second group included 132 healthy people among whom no history of psychiatric disorder was observed, either themselves or their intimate relatives. The participants of this group were selected from areas where the listed psychiatric hospitals were located.
The sampling was performed from a number of Psychiatric Hospitals in Tehran (Razi Educational and Therapeutic Psychiatric Center, Maymanat Psychiatric Hospital, Medical and Educational Center, Baghiatallah Hospital, Azadi Psychiatric Hospital, and Ayatollah Taleghani Educational hospital), and it lasted from November 2015 to December 2016. Participants in both groups were selected from the patients of Razi Educational and Therapeutic Psychiatric Center, Maymanat Psychiatric Hospital, Imam Hossein Medical and Educational Center, Baghiatallah Hospital, Azadi Psychiatric Hospital, and Ayatollah Taleghani Educational hospital using a convenience sampling method. The sampling was performed from November 2015 to December 2016. Both groups answered the questionnaires in a via-in-person method.
The exclusion criteria for the patient group were as follows: drug abuse or addiction, history of head injury or undergoing electroconvulsive therapy, psychotic disorders, or other physical disorders (chronic or acute). The exclusion criteria for the healthy group were as follows: first-degree relatives having BD or other psychiatric disorders and participants having physical disorders. We also tried to match the two groups in terms of demographic characteristics. Permission to conduct this study was obtained from the Psychosis Research Center of Razi Educational and Therapeutic Psychiatric Center. The study objectives were explained to the patients and their families and also to the healthy participants; their informed consents were obtained, and they were asked to complete the questionnaires.
Questionnaires
The Brief QoL.BD
This is a 12-item self-report tool developed by Michalak, Murray, and CREST [11] to assess the QoL of patients with BD. All items are rated on a 5-point Likert-type scale ranging from 1 (totally disagree) to 5 (totally agree), and the total score ranges from 12 to 60. Higher scores indicate better QoL. It must be noted that the original scale has been administered to a heterogeneous sample consisting of hospitalized and non-hospitalized participants, and two items, (Kept my home tidy) and (Travelled around freely), are more applicable to non-hospitalized individuals. Studies conducted by the original developers indicated good psychometric properties of the scale.
Cronbach’s alpha in a sample of 199 participants has been reported to be 0.87. Explanatory Factor Analysis (EFA) has shown a factor consisting of 12 items; test–retest reliability of the scale has been reported to be 0.69, and the scale has had moderate to high correlations with each one of the basic scales of the QoL.BD ranging from 0.51 (for spirituality) to 0.86 (for mood). Its concurrent validity was examined by calculating its correlation with the subscales of the SF-36. The correlation coefficient between the total score on the Brief QoL.BD and the general health factor of the SF-36 was found to be 0.63, and the correlations were also significant for the other subscales. In China, Xiao et al. [12] examined the content validity, test–retest reliability, and internal consistency of the Brief QoL.BD, and performed a CFA and item analysis for it. Results showed that Chinese version of the Brief QoL.BD had high internal consistency (Cronbach’s alpha 0.815) and test–retest reliability (0.808). In addition, confirmatory factor analysis (CFA) validated the original one-factor structure as well.
The SF-36
This is a non-disorder-specific QoL scale that has been utilized to assess QoL in patients with BD more than other scales. It has 6 subscales, including physical functioning, physical role functioning, bodily pain, general health perceptions, vitality, social role functioning, emotional role functioning, and mental health [13]. This scale was developed in the United States, and then its factorial validity was analyzed in 10 countries, including Denmark, Germany, Italy, Norway, Spain, Sweden, England, the United States, and the Netherlands. Results of CFA using structural equation modeling has indicated good construct validity in different countries. Brazier et al. [14] reported internal consistency coefficients of 0.72 to 0.93 and test–retest reliability estimates of 0.63–0.81 for the 8 subscales of the SF-36. In Iran, the validity and reliability of the SF-36 have been reported to be good [15]. In addition, in a study by Habibi et al. [16], the eight-dimensional factor structure of the SF-36 was supported, and its construct, criterion, and discriminatory validity estimates were reported to be good. Moreover, good internal consistency estimates have been reported for all the subscales of the SF-36 (all above 0.70). In this research, Cronbach’s alpha was found to be 0.79.
The BDI-II
This self-report instrument is widely used to assess the severity of depression-related signs and symptoms, and its 21 items have been developed based on observation of the typical symptoms of patients with depression [17]. The revised version of the BDI-II, compared to its first version, is more consistent with Diagnostic and Statistical Model of Mental Disorders (DSM-IV) and covers all components of depression according to cognitive theory [18]. Beck, Steer, and Garbin [19] showed that the second version like the previous one shows the existence and intensity of depressive symptoms in patients and normal individuals. They reported Cronbach’s alpha values of 0.86 and 0.81 in patient and non-patient samples, respectively. In Iran, Fata [20] administered the BDI-II to a sample of 94 individuals and reported a Cronbach’s alpha of 0.91 and a 1 week test–retest reliability of 0.94 for the scale. Another study in Iran, on a sample of 354 patients, has reported a Cronbach’s alpha of 0.913 [21]. In this study, the BDI-II was used to assess the severity of depression, and the Cronbach’s alpha was found to be 0.79.
The BRMAS
This 11-item scale developed in 1978 by Bech, Bowlig, Kramp, and Rafaelsen is used to assess the severity of mania and hypomania symptoms in the past 3 days. The total score of the BRMAS has been standardized, so the scores below 15 show hypomania, scores around 20 show moderate mania, and scores around 28 show severe mania. Studies have revealed that the simple sum of the 11 items is sufficient for examining the severity of manic states. The inter-observer reliability has been demonstrated to be high in studies conducted in some countries. The BRMAS has shown an acceptable external validity. In addition, it has good internal consistency (0.70) and reliability (0.73) [22]. In this study, the internal validity of this scale was found to be 0.70.
Statistical analysis
The study data were analyzed using SPSS v. 22 and LISREL statistical programs. First, participants’ demographic characteristics were assessed. Then, test–retest reliability, convergent validity, content validity, and internal consistency were examined.
3. Results
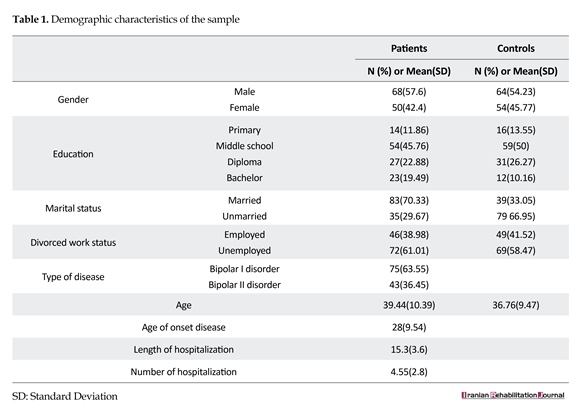
Table 1 shows the participants’ demographic characteristics. A total of 250 individuals participated in this study, among which 118 were patients with BD and 132 were healthy individuals. The mean(±SD) age of the patient and healthy groups was found to be 39.4(10.39) and 36.7(9.47) years, respectively. Among them, 54.8% of the participants in the patient group and 57.6% of the participants in the healthy group were males.
Content validity
Content validity refers to the extent to which items on a scale are relevant or comprehensive. Content Validity Index (CVI) is used to examine whether items on a scale are constructed to assess the focal construct. There are two forms of CVI: item-level (I-CVI) and scale-level (S-CVI) [13]. In order to assess content validity, four psychiatrists and two clinical psychologists with Ph.D. (other than the first author) were asked to assess the relevance of each item on a scale ranging from 1 (irrelevant) to 4 (very relevant). For each item, I-CVI referred to the number of experts who gave ratings of 3 or 4 to each item, and was divided by the number of all experts. S-CVI included S-CVI/UA (scale-level content validity index, universal agreement) and S-CVI-AVE (scale-level content validity index, average calculation method). The results indicated that I-CVI>0.77, S-CVI/UA>0.81, and S-CV. I/AVE>0.9, indicating a good content validity.
Confirmatory factor validity
In order to confirm the factors extracted from the Brief QoL.BD, confirmatory factor validity (CFA) was conducted using the LISREL software; the results of fit indices are presented in Table 2. The chi-squared test (χ2) is the most important fit index. This index shows the difference between the observed and estimated matrices. This index is very sensitive to the sample size; therefore, in large samples, it is divided by the degrees of freedom (df), and is considered to be good when its value is less than 2. But it is usually significant in samples consisting of more than 100 participants; therefore, it is not regarded as a proper goodness of fit index [23]. As you can see in the table, the chi-square value was found to be significant, but χ2/df ratio was found to be 1.90.
The estimated values indicate that the model has a relatively a good fit. Furthermore, χ2 was not found to be statistically significant. In addition, χ2/df ratio was found to be 1.90, which is consistent with the proposed criterion that a value less than 2 is appropriate. The adjusted goodness of fit index (AGFI), goodness of fit index (GFI), and comparative fit index (CFI) are in the range of 0–1; higher values for these indices indicate a better model fit [23]. The root mean square error of approximation (RMSEA)
Bipolar Disorder (BD) is a common psychiatric disorder. It emerges as a syndrome with a complete set of symptoms of mania and depression and affects 1–2.4% of adults and 2.5% of adolescents [1]. Diagnostic and Statistical Model of Mental Disorders (DSM-V) defines BD as a period of mania and depression. The period of mania is characterized by abnormally elevated mood—open or irritable—that lasts for a week. During the period of openness, purposeful activity or level of energy is abnormally and constantly high, which is observable for most of the day and nearly all days. In addition, this period is characterized by such symptoms as increased self-confidence, grandiosity, flight of ideas; distraction, talkativeness, and decreased need for sleep [2].
BD is a chronic disorder with a high rate of relapse, and even when undergoing pharmacological treatment, about 40–60% of the patients have at least one relapse into depression or mania within 2 years. Patients with BD are 15–20 times more likely to commit suicide than that of the general population. In addition, more than 15% of the patients with BD have made at least one suicidal attempt in their lives [3].
Various studies have also shown that BD imposes a great deal of social costs on patients and people around them due to their decreased productivity. BD has a lower recovery rate and a higher mortality rate than that of other mood disorders. In 2000, the world health organization (WHO) ranked BD as the sixth leading cause of lifetime adjustment impairment in people in the age group of 15–44 years throughout the world [4]. The most troubling problem for these patients is suicidal impulses. Low Quality of Life (QoL) along with interpersonal conflicts has been observed in these patients during recovery [5]. QoL refers to each individual’s range of needs related to his/her perception about feeling good. Good QoL is not the absence of illness, but it refers to feeling good about various social, physical, and psychological functions [6].
QoL is a multidimensional and complex concept encompassing objective and subjective factors related to an individual’s perception of life satisfaction, physical and family health, hope, social interactions, and psychological health [7]. WHO defines QoL as an individual’s perception of their position in life and according to the cultural setting, value systems, and condition of the person’s life in relation to his/her goals, expectations, and standards [8]. There is evidence showing that the destructive effects of BD on the QoL of these patients is so severe that even in the normal mood phase, these patients are more vulnerable to having problems in their social and career lives compared to the patients with multiple sclerosis (MS) and rheumatoid arthritis [9].
Given that QoL is one of the most important constructs in assessing people’s vulnerability to physical and psychological impairments, various instruments have been designed to assess it, including the 36-Item Short-Form Health Survey (SF-36) questionnaire developed by the WHO; however, it seems that each questionnaire highlights a particular aspect of QoL, such as spirituality or attachment and ignoring the other aspects [10]. In addition, SF-36 is primarily used to assess the general QoL of individuals, and it is used for a wide range of people from healthy individuals to those with various physical and psychological disorders. However, there are not many instruments available to assess disorder-related QoL, including for BD.
The Quality of Life in Bipolar Disorder (QoL.BD) was the first instrument developed to assess BD-related QoL, and because it is disorder-specific, it appears to have a significant sensitivity and specificity relative to other QoL instruments. It has 56 items and 13 subscales. Because patients with BD may experience difficulty in answering a large number of items, the developers of the QoL.BD designed a short form of the questionnaire—the Brief Quality of Life in Bipolar Disorder (Brief QoL.BD). Brief QoL.BD assesses different aspects of the life of patients with BD, and the developers reported its good psychometric properties [11]. This questionnaire has been translated into some languages. The Spanish version showed a high reliability (α=0.95) and its convergent validity was relatively good (correlation coefficients with SF-36 ranging from 0.453 and 0.819; P<0.01). The Chinese version also had very high internal consistency (α=0.95) and retest reliability (interclass correlation coefficient about 0.808)
Due to the fact that assessing the QoL of patients with BD is both important for the diagnosis and examination of treatment outcomes, and given to the lack of Persian disorder-specific scale to assess QoL, the aim of this study was to consider psychometric properties of the Persian version of Brief QoL.BD in patients with BD in psychiatric hospitals of Tehran.
2. Methods
Population, sample, and sampling method
The statistical population comprised all the patients and normal persons in Tehran. The sample population included 118 patients suffering from BD and 110 healthy subjects. The sampling was an available one and the sample size was edited based on Gross Guideline which allocates 5–10 subjects to each question. The study participants were in two groups: the patient group and the healthy group. The patient group was suffering from BD being hospitalized in psychiatric hospitals for 1.5 months while had reached a relatively stable condition by taking mood stabilizer medications and showing signs of recovery. To confirm this, the patients who obtained a score of less than 13 in Beck Depression Inventory-II (BDI-II) and less than 6 in Beck–Rafaelsen Mania Rating Scale (BRMAS) were selected for further study.
The sampling was performed from a number of psychiatric hospitals in Tehran (Razi Psychiatric Center, Meymanat Psychiatric Hospital, Imam Hossein Hospital, Taleghani Hospital, Baghiatallah Hospital, and Azadi Psychiatric Center), and it lasted from March to December 2016. The second group included 132 healthy people among whom no history of psychiatric disorder was observed, either themselves or their intimate relatives. The participants of this group were selected from areas where the listed psychiatric hospitals were located.
The sampling was performed from a number of Psychiatric Hospitals in Tehran (Razi Educational and Therapeutic Psychiatric Center, Maymanat Psychiatric Hospital, Medical and Educational Center, Baghiatallah Hospital, Azadi Psychiatric Hospital, and Ayatollah Taleghani Educational hospital), and it lasted from November 2015 to December 2016. Participants in both groups were selected from the patients of Razi Educational and Therapeutic Psychiatric Center, Maymanat Psychiatric Hospital, Imam Hossein Medical and Educational Center, Baghiatallah Hospital, Azadi Psychiatric Hospital, and Ayatollah Taleghani Educational hospital using a convenience sampling method. The sampling was performed from November 2015 to December 2016. Both groups answered the questionnaires in a via-in-person method.
The exclusion criteria for the patient group were as follows: drug abuse or addiction, history of head injury or undergoing electroconvulsive therapy, psychotic disorders, or other physical disorders (chronic or acute). The exclusion criteria for the healthy group were as follows: first-degree relatives having BD or other psychiatric disorders and participants having physical disorders. We also tried to match the two groups in terms of demographic characteristics. Permission to conduct this study was obtained from the Psychosis Research Center of Razi Educational and Therapeutic Psychiatric Center. The study objectives were explained to the patients and their families and also to the healthy participants; their informed consents were obtained, and they were asked to complete the questionnaires.
Questionnaires
The Brief QoL.BD
This is a 12-item self-report tool developed by Michalak, Murray, and CREST [11] to assess the QoL of patients with BD. All items are rated on a 5-point Likert-type scale ranging from 1 (totally disagree) to 5 (totally agree), and the total score ranges from 12 to 60. Higher scores indicate better QoL. It must be noted that the original scale has been administered to a heterogeneous sample consisting of hospitalized and non-hospitalized participants, and two items, (Kept my home tidy) and (Travelled around freely), are more applicable to non-hospitalized individuals. Studies conducted by the original developers indicated good psychometric properties of the scale.
Cronbach’s alpha in a sample of 199 participants has been reported to be 0.87. Explanatory Factor Analysis (EFA) has shown a factor consisting of 12 items; test–retest reliability of the scale has been reported to be 0.69, and the scale has had moderate to high correlations with each one of the basic scales of the QoL.BD ranging from 0.51 (for spirituality) to 0.86 (for mood). Its concurrent validity was examined by calculating its correlation with the subscales of the SF-36. The correlation coefficient between the total score on the Brief QoL.BD and the general health factor of the SF-36 was found to be 0.63, and the correlations were also significant for the other subscales. In China, Xiao et al. [12] examined the content validity, test–retest reliability, and internal consistency of the Brief QoL.BD, and performed a CFA and item analysis for it. Results showed that Chinese version of the Brief QoL.BD had high internal consistency (Cronbach’s alpha 0.815) and test–retest reliability (0.808). In addition, confirmatory factor analysis (CFA) validated the original one-factor structure as well.
The SF-36
This is a non-disorder-specific QoL scale that has been utilized to assess QoL in patients with BD more than other scales. It has 6 subscales, including physical functioning, physical role functioning, bodily pain, general health perceptions, vitality, social role functioning, emotional role functioning, and mental health [13]. This scale was developed in the United States, and then its factorial validity was analyzed in 10 countries, including Denmark, Germany, Italy, Norway, Spain, Sweden, England, the United States, and the Netherlands. Results of CFA using structural equation modeling has indicated good construct validity in different countries. Brazier et al. [14] reported internal consistency coefficients of 0.72 to 0.93 and test–retest reliability estimates of 0.63–0.81 for the 8 subscales of the SF-36. In Iran, the validity and reliability of the SF-36 have been reported to be good [15]. In addition, in a study by Habibi et al. [16], the eight-dimensional factor structure of the SF-36 was supported, and its construct, criterion, and discriminatory validity estimates were reported to be good. Moreover, good internal consistency estimates have been reported for all the subscales of the SF-36 (all above 0.70). In this research, Cronbach’s alpha was found to be 0.79.
The BDI-II
This self-report instrument is widely used to assess the severity of depression-related signs and symptoms, and its 21 items have been developed based on observation of the typical symptoms of patients with depression [17]. The revised version of the BDI-II, compared to its first version, is more consistent with Diagnostic and Statistical Model of Mental Disorders (DSM-IV) and covers all components of depression according to cognitive theory [18]. Beck, Steer, and Garbin [19] showed that the second version like the previous one shows the existence and intensity of depressive symptoms in patients and normal individuals. They reported Cronbach’s alpha values of 0.86 and 0.81 in patient and non-patient samples, respectively. In Iran, Fata [20] administered the BDI-II to a sample of 94 individuals and reported a Cronbach’s alpha of 0.91 and a 1 week test–retest reliability of 0.94 for the scale. Another study in Iran, on a sample of 354 patients, has reported a Cronbach’s alpha of 0.913 [21]. In this study, the BDI-II was used to assess the severity of depression, and the Cronbach’s alpha was found to be 0.79.
The BRMAS
This 11-item scale developed in 1978 by Bech, Bowlig, Kramp, and Rafaelsen is used to assess the severity of mania and hypomania symptoms in the past 3 days. The total score of the BRMAS has been standardized, so the scores below 15 show hypomania, scores around 20 show moderate mania, and scores around 28 show severe mania. Studies have revealed that the simple sum of the 11 items is sufficient for examining the severity of manic states. The inter-observer reliability has been demonstrated to be high in studies conducted in some countries. The BRMAS has shown an acceptable external validity. In addition, it has good internal consistency (0.70) and reliability (0.73) [22]. In this study, the internal validity of this scale was found to be 0.70.
Statistical analysis
The study data were analyzed using SPSS v. 22 and LISREL statistical programs. First, participants’ demographic characteristics were assessed. Then, test–retest reliability, convergent validity, content validity, and internal consistency were examined.
3. Results
Table 1 shows the participants’ demographic characteristics. A total of 250 individuals participated in this study, among which 118 were patients with BD and 132 were healthy individuals. The mean(±SD) age of the patient and healthy groups was found to be 39.4(10.39) and 36.7(9.47) years, respectively. Among them, 54.8% of the participants in the patient group and 57.6% of the participants in the healthy group were males.
Content validity
Content validity refers to the extent to which items on a scale are relevant or comprehensive. Content Validity Index (CVI) is used to examine whether items on a scale are constructed to assess the focal construct. There are two forms of CVI: item-level (I-CVI) and scale-level (S-CVI) [13]. In order to assess content validity, four psychiatrists and two clinical psychologists with Ph.D. (other than the first author) were asked to assess the relevance of each item on a scale ranging from 1 (irrelevant) to 4 (very relevant). For each item, I-CVI referred to the number of experts who gave ratings of 3 or 4 to each item, and was divided by the number of all experts. S-CVI included S-CVI/UA (scale-level content validity index, universal agreement) and S-CVI-AVE (scale-level content validity index, average calculation method). The results indicated that I-CVI>0.77, S-CVI/UA>0.81, and S-CV. I/AVE>0.9, indicating a good content validity.
Confirmatory factor validity
In order to confirm the factors extracted from the Brief QoL.BD, confirmatory factor validity (CFA) was conducted using the LISREL software; the results of fit indices are presented in Table 2. The chi-squared test (χ2) is the most important fit index. This index shows the difference between the observed and estimated matrices. This index is very sensitive to the sample size; therefore, in large samples, it is divided by the degrees of freedom (df), and is considered to be good when its value is less than 2. But it is usually significant in samples consisting of more than 100 participants; therefore, it is not regarded as a proper goodness of fit index [23]. As you can see in the table, the chi-square value was found to be significant, but χ2/df ratio was found to be 1.90.
The estimated values indicate that the model has a relatively a good fit. Furthermore, χ2 was not found to be statistically significant. In addition, χ2/df ratio was found to be 1.90, which is consistent with the proposed criterion that a value less than 2 is appropriate. The adjusted goodness of fit index (AGFI), goodness of fit index (GFI), and comparative fit index (CFI) are in the range of 0–1; higher values for these indices indicate a better model fit [23]. The root mean square error of approximation (RMSEA)
was found to be equal to 0.065, which is considered as satisfactory. According to the above indices, we can conclude that the model has a relatively good fit.
Convergent validity
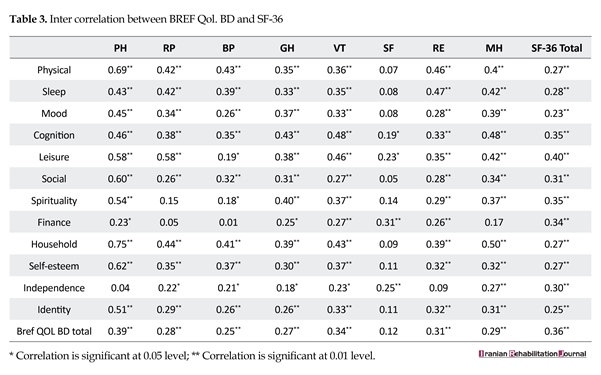
SF-36 was used to examine convergent validity. Spearman’s correlation coefficient was calculated between the scores on this questionnaire and those on the SCS; the results are presented in Table 3. According to the results, there are significant correlations between the items of this questionnaire and subscales of SF-36.
Discriminant validity
In order to examine discriminant validity of the Brief QoL.BD, this questionnaire was tested on patient and healthy groups to determine whether there was a significant difference between them in terms of their total scores on the scale; in other words, we intended to test whether Brief QoL.BD can distinguish between patients and healthy individuals. The results of independent t-test indicated a significant difference between the patients and healthy participants (P<0.01); this indicated the good discriminant validity of the Brief QoL.BD.
Internal consistency
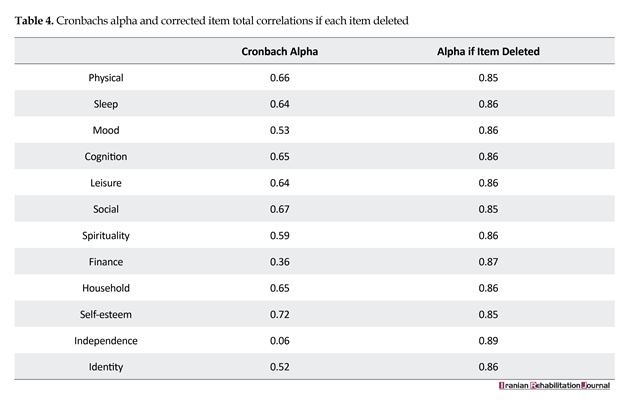
The internal consistency of the Brief QoL.BD was examined using the Cronbach’s alpha coefficient; an alpha value of 0.87 was obtained. In addition, inter-item and item-total correlation estimates ranged from 0.025 to 0.62 and 0.52 to 0.72, respectively; this indicated a good consistency among items. In addition, the alpha value was not increased by removing any item, and by removing any item, it still ranged from 0.86 to 0.77. Table 4 shows the internal consistency of the questionnaire.
Convergent validity
SF-36 was used to examine convergent validity. Spearman’s correlation coefficient was calculated between the scores on this questionnaire and those on the SCS; the results are presented in Table 3. According to the results, there are significant correlations between the items of this questionnaire and subscales of SF-36.
Discriminant validity
In order to examine discriminant validity of the Brief QoL.BD, this questionnaire was tested on patient and healthy groups to determine whether there was a significant difference between them in terms of their total scores on the scale; in other words, we intended to test whether Brief QoL.BD can distinguish between patients and healthy individuals. The results of independent t-test indicated a significant difference between the patients and healthy participants (P<0.01); this indicated the good discriminant validity of the Brief QoL.BD.
Internal consistency
The internal consistency of the Brief QoL.BD was examined using the Cronbach’s alpha coefficient; an alpha value of 0.87 was obtained. In addition, inter-item and item-total correlation estimates ranged from 0.025 to 0.62 and 0.52 to 0.72, respectively; this indicated a good consistency among items. In addition, the alpha value was not increased by removing any item, and by removing any item, it still ranged from 0.86 to 0.77. Table 4 shows the internal consistency of the questionnaire.
Test-retest reliability
Ten days after the patients provided their first set of responses, a total of 30 patients were asked to provide their responses for the second time on the Brief QoL.BD questionnaire. Then, we calculated the Pearson’s correlation coefficient between the first and the second set of scores of the scale; a correlation of 0.84 was found that indicated a high test-retest reliability (P<0.01)
4. Discussion
Given to the fact that QoL is a very important construct in assessing the recovery of patients with mental disorders, including those with BD, and due to the lack of proper instruments for assessing QoL of patents with BD, we aimed to examine the psychometric properties of the Brief QoL.BD among patients with BD. Therefore, forward and backward translation of Brief QoL.BD was performed; its content validity was examined by several experts, and a CFA was undertaken. Consistent with the findings of the developers of the original scale, the findings of this study indicated good content validity of the scale.
Ten days after the patients provided their first set of responses, a total of 30 patients were asked to provide their responses for the second time on the Brief QoL.BD questionnaire. Then, we calculated the Pearson’s correlation coefficient between the first and the second set of scores of the scale; a correlation of 0.84 was found that indicated a high test-retest reliability (P<0.01)
4. Discussion
Given to the fact that QoL is a very important construct in assessing the recovery of patients with mental disorders, including those with BD, and due to the lack of proper instruments for assessing QoL of patents with BD, we aimed to examine the psychometric properties of the Brief QoL.BD among patients with BD. Therefore, forward and backward translation of Brief QoL.BD was performed; its content validity was examined by several experts, and a CFA was undertaken. Consistent with the findings of the developers of the original scale, the findings of this study indicated good content validity of the scale.
The results of CFA also showed the applicability of the factor structure of the Persian version of the Brief QoL.BD in Iranian population. In other words, the fit indices were found to be acceptable. This finding agrees with the results of a study by Michalak et al. [12] and also the findings of Xiao et al. [13]. But in Persian version, the CFA indices were found to be lower than that of the main version and Chinese one. This was due to the situation of patients such as the time and the place where they answered. Some of them did not recognize items correctly and needed extra explanation and some of them did not answer seriously and replied randomly to items. Although such questions were omitted in the analysis, errors must have crept in.
To date, there are no instruments to assess the QoL of patients with BD; therefore, we used the SF-36 to examine the convergent validity of the Brief QoL.BD. Our results indicated relatively good correlations between each item of the Brief QoL.BD and each subscale of the SF-36 except some of the social function subscale in SF-36. This is to some extent along of the content of items in social function in SF-36 that are different compared to brief QoL.BD specialized to BD. In case of inpatients with BD, social function is strongly impaired.
The discriminant validity of the scale was also examined by comparing the total scores of patient and healthy groups. The results indicated that the scale could distinguish between patient and healthy individuals (t=38.64). This finding is also consistent with the findings of the original developers [12] and also what was found with the Chinese version of the scale [13].
BD interferes all aspect of mental and physical health. Patients in psychiatric centers do not have enough physical activity, experience unstable mood, and deficits in sleep and eating. Due to mood instability, they do not have a good concentration; their self-esteem decreases, and they become dependent on others. These are the outcomes of BD which lowers their quality of life. Therefore, it is predictable that the QoL.BD scores are significantly different in patient and normal persons. Thus, the scale could differentiate both groups properly.
We used Cronbach’s alpha coefficient to examine internal consistency. The alpha estimates for the extracted factors and the total scale were in an acceptable range, indicating relatively high internal consistency of the scale. All items also had acceptable alpha estimates. This finding is in line with what has been reported by the original developers [12], and also what has been found with the Chinese version of the scale [13].
Test–retest reliability was examined through a second examination of the scale on a group of participants 10 days after the first administration, which was found to be 0.89 (P<0.01), indicating very high reliability of the scale. Finally, the scale has high validity and reliability in Iranian population, and given that its items are compatible with Iranian culture, it can be used as a valid tool to assess QoL of patients with BD.
Like any other study, this study had some limitations. First, the instrument examined in this study is a self-report scale; therefore, it can involve biases when answered by patients with BD. The second limitation concerns the sample size that limits the generalizability of the results. Given that patients with BD who meet inclusion criteria are difficult to find, the sampling procedure took a long time and resulted in a small sample. In addition, the study participants were all recruited from Tehran; this can also make problems for the generalizability of the results. Therefore, future studies are suggested to use family-report instruments, work with larger samples, and conduct the sampling in different locations.
5. Conclusion
This study showed that the Persian version of Brief QoL BD has acceptable psychometric properties in bipolar patients in Iran and can be used as an effective instrument for assessing the quality of life in these patients. Since the quality of life is an important and unassailable factor in assessing pharmacological and psychological intervention outcome, this scale which is disorder-specific is a useful tool for this purpose and can be used in different researches and therapeutic fields.
Acknowledgments
This study was supported as a research project by Psychosis research Center in University of Social Welfare and Rehabilitation Sciences (grant number: 1238). I hereby wish to thank and appreciate the management, honorable vice, and hospital staff for their committed assistance along various stages of this research as well as all participants of the study.
Conflict of Interest
The authors declare that there is no conflicts of interest regarding the publication of this paper. Fereshte Momeni designed the study and did literature search and clinical and experimental studies and also prepared the manuscript. Yasaman Shiyasi and Hosna Mirzaasgari did data acquisition and data analysis. All of the authors revised the manuscript.
References
[1]Gomes BC, Kleinman A, Ferrari Carvalho A, Pereira TCF, Gurgel AP, et al. Quality of life in youth with bipolar disorder and unaffected offspring of parents with bipolar disorder. Journal of Affective Disorder. 2016; 202:53-7. doi: 10.1016/j.jad.2016.05.041
[2]Sadock BJ, Sadock VA. Kaplan and Sadock’ synopsis of psychiatry: Behavioral Sciences/clinical psychiatry. Philadelphia: Lippincott Williams & Wilkins; 2007.
[3]Miklowitz DJ, Johnson SL. The psychopathology and treatment of bipolar disorder. Annual Review of Clinical Psychology. 2006; 2(1):199-235. doi: 10.1146/annurev.clinpsy.2.022305.095332
[4]Havermans R, Nicolson NA, Devries MW. Daily hassles, uplifts,and time use in individuals with bipolar disorder remission. The Journal of Nervous and Mental Disease. 2007; 195(9):745-51. doi: 10.1097/nmd.0b013e318142cbf0
[5]Fulford D, Peckham AD, Johnson K, Johnson SL. Emotion perception and quality of life in bipolar I disorder. Journal of Affective Disorders. 2014; 152-154:491–7. doi: 10.1016/j.jad.2013.08.034
[6]Morton E, Murray G. What does quality of life refer to in bipolar disorder research? A systematic review of the construct’s definition,usage and measurement. Journal of Affective Disorder. 2017; 212:128-37. doi: 10.1016/j.jad.2017.01.026
[7]Pennacchini M, Bertolaso M, Elvira MM, De Marinis MG. A Brief history of the quality of Life: Its use in medicine and in philosophy. Clinical Therapeutics. 2011; 162(3):e99-e103. PMID: 21717042
[8]Cummins R. The domains of life satisfaction: An attempt to order chaos. Social Indicators Research. 1996; 38(3):303-28. doi: 10.1007/bf00292050
[9]World Health Organization. The World Health Organization Quality of Life assessment(WHOQL):Position paper from the World Health organization. Social Science & Medicine. 1995; 41(10):1403-9. doi: 1016/0277-9536(95)00112-k
[10]Michalak EE, Yatham LN, Lam RW. Quality of life in bipolar disorder:A review of the literature. Health and Quality of Life Outcomes. 2005; 3(1):72. doi: 10.1186/1477-7525-3-72
[11]Michalak E, .E, Yatham LN, Kolesar S, Lam RW. Bipolar disorder and quality of life: A patient-centered perspective. Quality of life research. 2006; 15(1):25-37. doi: 10.1007/s11136-005-0376-7
[12]Michalak EE, Murray G, Crest BD. Development of the QoL.BD: A disorder-spesific scale to assess quality of life in bipolar disorder. Bipolar Disorders. 2010; 12(7):727-40. doi: 10.1111/j.1399-5618.2010.00865.x
[13]Xiao L, Gao Y, Zhang L, Chen P, Sun X, Tang S. Validity and reliability of the Brief version of Quality of Life in Bipolar disorder (Bref QoL.BD) among Chinese bipolar patients. Journal of Affective Disorders. 2016; 193:66-77. doi: 10.1016/j.jad.2015.12.074
[14]Kline Leidy N. Health-related quality of life assessment in euthymic and depressed patients with bipolar disorder Psychometric performance of four self-report measures. Journal of Affective Disorders. 1998; 48(2-3):207–14. doi: 10.1016/s0165-0327(97)00147-x
[15]Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ. 1992; 305(6846):160-4. doi: 10.1136/bmj.305.6846.160
[16]Montazeri A, Goshtasebi A, Vahdanian M, Gandek B. The short form Health survey (SF-36): Translation and validation study of the Iranian version. Quality of Life Research. 2005; 14(3):875-82. doi: 10.1007/s11136-004-1014-5
[17]Habibi M, Khodaei E, Moghadamzadeh A, Shamsedini S, Barekatain M. [Psychometric properties and hierarchical factor structure of short form health survey scale(SF-36) in a non-clinical sample (Persian)]. Journal of research in Behavioral sciences. 2010; 10(6): 472-90.
[18]Beck AT, Streer RA. Beck Depression Inventory. New York: The psychological corporation; 1993.
[19]Beck AT, Streer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1998; 8(1):77-100. doi: 10.1016/0272-7358(88)90050-5
[20]Fata L, Birashk B, Atefvahid MK, Dabson KS. [Meaning assignment structures/schema, emotional states and cognitive processing of emotional information: comparing two conceptual frameworks (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology. 2005; 11(3):312-26.
[21]Stefan-Dabson K, Mohammadkhani P, Massah-Choulabi O. [Psychometrics characteristic of Beck Depression Inventory-II in patients with magor depressive disorder (Persian)]. Archives of Rehabilitation. 2007; 8:80-6.
[22]Bech P, Bolwig TG, Kramp P, Rafalsen OJ. The bech-rafaelsen mania scale and the Hamilton depression scale. Acta Psychiatrica Scandinavica. 1979; 59(4):420-30. PMID: 433633
[23]Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980; 88(3):588-606. doi: 10.1037//0033-2909.88.3.588
To date, there are no instruments to assess the QoL of patients with BD; therefore, we used the SF-36 to examine the convergent validity of the Brief QoL.BD. Our results indicated relatively good correlations between each item of the Brief QoL.BD and each subscale of the SF-36 except some of the social function subscale in SF-36. This is to some extent along of the content of items in social function in SF-36 that are different compared to brief QoL.BD specialized to BD. In case of inpatients with BD, social function is strongly impaired.
The discriminant validity of the scale was also examined by comparing the total scores of patient and healthy groups. The results indicated that the scale could distinguish between patient and healthy individuals (t=38.64). This finding is also consistent with the findings of the original developers [12] and also what was found with the Chinese version of the scale [13].
BD interferes all aspect of mental and physical health. Patients in psychiatric centers do not have enough physical activity, experience unstable mood, and deficits in sleep and eating. Due to mood instability, they do not have a good concentration; their self-esteem decreases, and they become dependent on others. These are the outcomes of BD which lowers their quality of life. Therefore, it is predictable that the QoL.BD scores are significantly different in patient and normal persons. Thus, the scale could differentiate both groups properly.
We used Cronbach’s alpha coefficient to examine internal consistency. The alpha estimates for the extracted factors and the total scale were in an acceptable range, indicating relatively high internal consistency of the scale. All items also had acceptable alpha estimates. This finding is in line with what has been reported by the original developers [12], and also what has been found with the Chinese version of the scale [13].
Test–retest reliability was examined through a second examination of the scale on a group of participants 10 days after the first administration, which was found to be 0.89 (P<0.01), indicating very high reliability of the scale. Finally, the scale has high validity and reliability in Iranian population, and given that its items are compatible with Iranian culture, it can be used as a valid tool to assess QoL of patients with BD.
Like any other study, this study had some limitations. First, the instrument examined in this study is a self-report scale; therefore, it can involve biases when answered by patients with BD. The second limitation concerns the sample size that limits the generalizability of the results. Given that patients with BD who meet inclusion criteria are difficult to find, the sampling procedure took a long time and resulted in a small sample. In addition, the study participants were all recruited from Tehran; this can also make problems for the generalizability of the results. Therefore, future studies are suggested to use family-report instruments, work with larger samples, and conduct the sampling in different locations.
5. Conclusion
This study showed that the Persian version of Brief QoL BD has acceptable psychometric properties in bipolar patients in Iran and can be used as an effective instrument for assessing the quality of life in these patients. Since the quality of life is an important and unassailable factor in assessing pharmacological and psychological intervention outcome, this scale which is disorder-specific is a useful tool for this purpose and can be used in different researches and therapeutic fields.
Acknowledgments
This study was supported as a research project by Psychosis research Center in University of Social Welfare and Rehabilitation Sciences (grant number: 1238). I hereby wish to thank and appreciate the management, honorable vice, and hospital staff for their committed assistance along various stages of this research as well as all participants of the study.
Conflict of Interest
The authors declare that there is no conflicts of interest regarding the publication of this paper. Fereshte Momeni designed the study and did literature search and clinical and experimental studies and also prepared the manuscript. Yasaman Shiyasi and Hosna Mirzaasgari did data acquisition and data analysis. All of the authors revised the manuscript.
References
[1]Gomes BC, Kleinman A, Ferrari Carvalho A, Pereira TCF, Gurgel AP, et al. Quality of life in youth with bipolar disorder and unaffected offspring of parents with bipolar disorder. Journal of Affective Disorder. 2016; 202:53-7. doi: 10.1016/j.jad.2016.05.041
[2]Sadock BJ, Sadock VA. Kaplan and Sadock’ synopsis of psychiatry: Behavioral Sciences/clinical psychiatry. Philadelphia: Lippincott Williams & Wilkins; 2007.
[3]Miklowitz DJ, Johnson SL. The psychopathology and treatment of bipolar disorder. Annual Review of Clinical Psychology. 2006; 2(1):199-235. doi: 10.1146/annurev.clinpsy.2.022305.095332
[4]Havermans R, Nicolson NA, Devries MW. Daily hassles, uplifts,and time use in individuals with bipolar disorder remission. The Journal of Nervous and Mental Disease. 2007; 195(9):745-51. doi: 10.1097/nmd.0b013e318142cbf0
[5]Fulford D, Peckham AD, Johnson K, Johnson SL. Emotion perception and quality of life in bipolar I disorder. Journal of Affective Disorders. 2014; 152-154:491–7. doi: 10.1016/j.jad.2013.08.034
[6]Morton E, Murray G. What does quality of life refer to in bipolar disorder research? A systematic review of the construct’s definition,usage and measurement. Journal of Affective Disorder. 2017; 212:128-37. doi: 10.1016/j.jad.2017.01.026
[7]Pennacchini M, Bertolaso M, Elvira MM, De Marinis MG. A Brief history of the quality of Life: Its use in medicine and in philosophy. Clinical Therapeutics. 2011; 162(3):e99-e103. PMID: 21717042
[8]Cummins R. The domains of life satisfaction: An attempt to order chaos. Social Indicators Research. 1996; 38(3):303-28. doi: 10.1007/bf00292050
[9]World Health Organization. The World Health Organization Quality of Life assessment(WHOQL):Position paper from the World Health organization. Social Science & Medicine. 1995; 41(10):1403-9. doi: 1016/0277-9536(95)00112-k
[10]Michalak EE, Yatham LN, Lam RW. Quality of life in bipolar disorder:A review of the literature. Health and Quality of Life Outcomes. 2005; 3(1):72. doi: 10.1186/1477-7525-3-72
[11]Michalak E, .E, Yatham LN, Kolesar S, Lam RW. Bipolar disorder and quality of life: A patient-centered perspective. Quality of life research. 2006; 15(1):25-37. doi: 10.1007/s11136-005-0376-7
[12]Michalak EE, Murray G, Crest BD. Development of the QoL.BD: A disorder-spesific scale to assess quality of life in bipolar disorder. Bipolar Disorders. 2010; 12(7):727-40. doi: 10.1111/j.1399-5618.2010.00865.x
[13]Xiao L, Gao Y, Zhang L, Chen P, Sun X, Tang S. Validity and reliability of the Brief version of Quality of Life in Bipolar disorder (Bref QoL.BD) among Chinese bipolar patients. Journal of Affective Disorders. 2016; 193:66-77. doi: 10.1016/j.jad.2015.12.074
[14]Kline Leidy N. Health-related quality of life assessment in euthymic and depressed patients with bipolar disorder Psychometric performance of four self-report measures. Journal of Affective Disorders. 1998; 48(2-3):207–14. doi: 10.1016/s0165-0327(97)00147-x
[15]Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ. 1992; 305(6846):160-4. doi: 10.1136/bmj.305.6846.160
[16]Montazeri A, Goshtasebi A, Vahdanian M, Gandek B. The short form Health survey (SF-36): Translation and validation study of the Iranian version. Quality of Life Research. 2005; 14(3):875-82. doi: 10.1007/s11136-004-1014-5
[17]Habibi M, Khodaei E, Moghadamzadeh A, Shamsedini S, Barekatain M. [Psychometric properties and hierarchical factor structure of short form health survey scale(SF-36) in a non-clinical sample (Persian)]. Journal of research in Behavioral sciences. 2010; 10(6): 472-90.
[18]Beck AT, Streer RA. Beck Depression Inventory. New York: The psychological corporation; 1993.
[19]Beck AT, Streer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1998; 8(1):77-100. doi: 10.1016/0272-7358(88)90050-5
[20]Fata L, Birashk B, Atefvahid MK, Dabson KS. [Meaning assignment structures/schema, emotional states and cognitive processing of emotional information: comparing two conceptual frameworks (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology. 2005; 11(3):312-26.
[21]Stefan-Dabson K, Mohammadkhani P, Massah-Choulabi O. [Psychometrics characteristic of Beck Depression Inventory-II in patients with magor depressive disorder (Persian)]. Archives of Rehabilitation. 2007; 8:80-6.
[22]Bech P, Bolwig TG, Kramp P, Rafalsen OJ. The bech-rafaelsen mania scale and the Hamilton depression scale. Acta Psychiatrica Scandinavica. 1979; 59(4):420-30. PMID: 433633
[23]Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980; 88(3):588-606. doi: 10.1037//0033-2909.88.3.588
Article type: Original Research Articles |
Subject:
Psychology
Received: 2017/05/28 | Accepted: 2017/07/19 | Published: 2017/10/2
Received: 2017/05/28 | Accepted: 2017/07/19 | Published: 2017/10/2
References
1. Gomes BC, Kleinman A, Ferrari Carvalho A, Pereira TCF, Gurgel AP, et al. Quality of life in youth with bipolar disorder and unaffected offspring of parents with bipolar disorder. Journal of Affective Disorder. 2016; 202:53-7. doi: 10.1016/j.jad.2016.05.041 [DOI:10.1016/j.jad.2016.05.041]
2. Sadock BJ, Sadock VA. Kaplan and Sadock' synopsis of psychiatry: Behavioral Sciences/clinical psychiatry. Philadelphia: Lippincott Williams & Wilkins; 2007.
3. Miklowitz DJ, Johnson SL. The psychopathology and treatment of bipolar disorder. Annual Review of Clinical Psychology. 2006; 2(1):199-235. doi: 10.1146/annurev.clinpsy.2.022305.095332 [DOI:10.1146/annurev.clinpsy.2.022305.095332]
4. Havermans R, Nicolson NA, Devries MW. Daily hassles, uplifts,and time use in individuals with bipolar disorder remission. The Journal of Nervous and Mental Disease. 2007; 195(9):745-51. doi: 10.1097/nmd.0b013e318142cbf0 [DOI:10.1097/NMD.0b013e318142cbf0]
5. Fulford D, Peckham AD, Johnson K, Johnson SL. Emotion perception and quality of life in bipolar I disorder. Journal of Affective Disorders. 2014; 152-154:491–7. doi: 10.1016/j.jad.2013.08.034 [DOI:10.1016/j.jad.2013.08.034]
6. Morton E, Murray G. What does quality of life refer to in bipolar disorder research? A systematic review of the construct's definition,usage and measurement. Journal of Affective Disorder. 2017; 212:128-37. doi: 10.1016/j.jad.2017.01.026 [DOI:10.1016/j.jad.2017.01.026]
7. Pennacchini M, Bertolaso M, Elvira MM, De Marinis MG. A Brief history of the quality of Life: Its use in medicine and in philosophy. Clinical Therapeutics. 2011; 162(3):e99-e103. PMID: 21717042
8. Cummins R. The domains of life satisfaction: An attempt to order chaos. Social Indicators Research. 1996; 38(3):303-28. doi: 10.1007/bf00292050 [DOI:10.1007/BF00292050]
9. World Health Organization. The World Health Organization Quality of Life assessment(WHOQL):Position paper from the World Health organization. Social Science & Medicine. 1995; 41(10):1403-9. doi: 1016/0277-9536(95)00112-k
10. Michalak EE, Yatham LN, Lam RW. Quality of life in bipolar disorder:A review of the literature. Health and Quality of Life Outcomes. 2005; 3(1):72. doi: 10.1186/1477-7525-3-72 [DOI:10.1186/1477-7525-3-72]
11. Michalak E, .E, Yatham LN, Kolesar S, Lam RW. Bipolar disorder and quality of life: A patient-centered perspective. Quality of life research. 2006; 15(1):25-37. doi: 10.1007/s11136-005-0376-7 [DOI:10.1007/s11136-005-0376-7]
12. Michalak EE, Murray G, Crest BD. Development of the QoL.BD: A disorder-spesific scale to assess quality of life in bipolar disorder. Bipolar Disorders. 2010; 12(7):727-40. doi: 10.1111/j.1399-5618.2010.00865.x [DOI:10.1111/j.1399-5618.2010.00865.x]
13. Xiao L, Gao Y, Zhang L, Chen P, Sun X, Tang S. Validity and reliability of the Brief version of Quality of Life in Bipolar disorder (Bref QoL.BD) among Chinese bipolar patients. Journal of Affective Disorders. 2016; 193:66-77. doi: 10.1016/j.jad.2015.12.074 [DOI:10.1016/j.jad.2015.12.074]
14. Kline Leidy N. Health-related quality of life assessment in euthymic and depressed patients with bipolar disorder Psychometric performance of four self-report measures. Journal of Affective Disorders. 1998; 48(2-3):207–14. doi: 10.1016/s0165-0327(97)00147-x [DOI:10.1016/S0165-0327(97)00147-X]
15. Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ. 1992; 305(6846):160-4. doi: 10.1136/bmj.305.6846.160 [DOI:10.1136/bmj.305.6846.160]
16. Montazeri A, Goshtasebi A, Vahdanian M, Gandek B. The short form Health survey (SF-36): Translation and validation study of the Iranian version. Quality of Life Research. 2005; 14(3):875-82. doi: 10.1007/s11136-004-1014-5 [DOI:10.1007/s11136-004-1014-5]
17. Habibi M, Khodaei E, Moghadamzadeh A, Shamsedini S, Barekatain M. [Psychometric properties and hierarchical factor structure of short form health survey scale(SF-36) in a non-clinical sample (Persian)]. Journal of research in Behavioral sciences. 2010; 10(6): 472-90.
18. Beck AT, Streer RA. Beck Depression Inventory. New York: The psychological corporation; 1993.
19. Beck AT, Streer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1998; 8(1):77-100. doi: 10.1016/0272-7358(88)90050-5 [DOI:10.1016/0272-7358(88)90050-5]
20. Fata L, Birashk B, Atefvahid MK, Dabson KS. [Meaning assignment structures/schema, emotional states and cognitive processing of emotional information: comparing two conceptual frameworks (Persian)]. Iranian Journal of Psychiatry and Clinical Psychology. 2005; 11(3):312-26.
21. Stefan-Dabson K, Mohammadkhani P, Massah-Choulabi O. [Psychometrics characteristic of Beck Depression Inventory-II in patients with magor depressive disorder (Persian)]. Archives of Rehabilitation. 2007; 8:80-6.
22. Bech P, Bolwig TG, Kramp P, Rafalsen OJ. The bech-rafaelsen mania scale and the Hamilton depression scale. Acta Psychiatrica Scandinavica. 1979; 59(4):420-30. PMID: 433633 [DOI:10.1111/j.1600-0447.1979.tb04484.x] [PMID]
23. Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980; 88(3):588-606. doi: 10.1037//0033-2909.88.3.588 [DOI:10.1037//0033-2909.88.3.588]
Send email to the article author