Volume 17, Issue 4 (December 2019)
Iranian Rehabilitation Journal 2019, 17(4): 377-384 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ghasemzadeh H, Akbari Kamrani A, Abolfathi Momtaz Y, Rassafiani M, Nourhashemi F, Sahaf R. Medical Risk Factors for Dementia; A Case-Control Study. Iranian Rehabilitation Journal 2019; 17 (4) :377-384
URL: http://irj.uswr.ac.ir/article-1-993-en.html
URL: http://irj.uswr.ac.ir/article-1-993-en.html
Hossein Ghasemzadeh *1 

 , Ahmadali Akbari Kamrani1
, Ahmadali Akbari Kamrani1 

 , Yadollah Abolfathi Momtaz1
, Yadollah Abolfathi Momtaz1 

 , Mehdi Rassafiani1
, Mehdi Rassafiani1 

 , Fatemeh Nourhashemi1
, Fatemeh Nourhashemi1 

 , Robab Sahaf2
, Robab Sahaf2 




 , Ahmadali Akbari Kamrani1
, Ahmadali Akbari Kamrani1 

 , Yadollah Abolfathi Momtaz1
, Yadollah Abolfathi Momtaz1 

 , Mehdi Rassafiani1
, Mehdi Rassafiani1 

 , Fatemeh Nourhashemi1
, Fatemeh Nourhashemi1 

 , Robab Sahaf2
, Robab Sahaf2 


1- Department of Ageing, Research Center on Ageing, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Department of Occupational Therapy, Faculty of Allied Health Sciences, Kuwait University, Kuwait.
2- Department of Occupational Therapy, Faculty of Allied Health Sciences, Kuwait University, Kuwait.
Full-Text [PDF 598 kb]
(1540 Downloads)
| Abstract (HTML) (3885 Views)
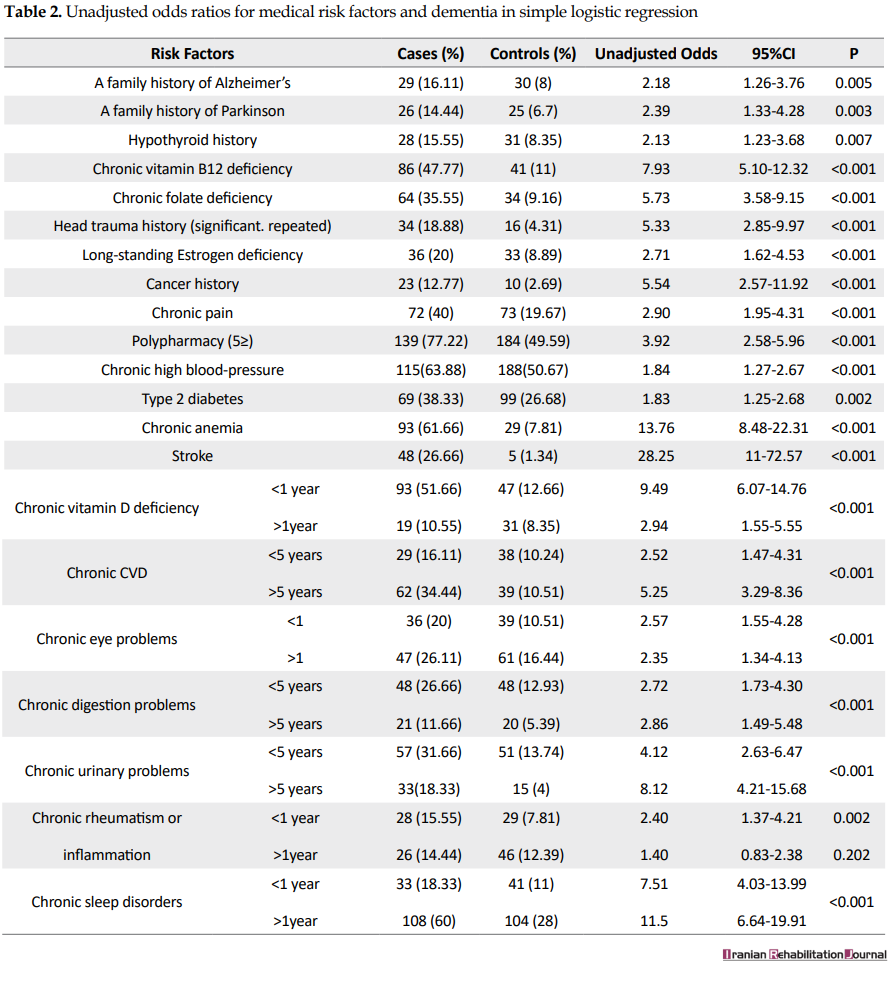
As it is presented in Table 2, the univariate logistic regression demonstrated that a history of all the medical problems under assessment had significantly increased the chances of dementia. However, because of the high number of variables, we did not include insignificant variables such as homocysteine deficiency, history of high blood lipids, and open-heart operation in Table 2.
Table 3 presents the results of multiple logistic regression after adjusting for age, sex, and education. A history of cancer (OR=15.31, P<0.001) and chronic anemia (OR=5.95, P<0.001) had significantly increased the chances of dementia. Moreover, a history of less (OR=7.50, P<0.001) and more than (OR=4.73, P<0.001) 1 year of vitamin D3 deficiency had also significantly increased the risk of dementia for the individuals.
4. Discussion
In the present study, we evaluated the association between dementia and medical risk factors. Although previous studies have reported different risk factors for dementia, but as far as we know, it is the first time that the risk factors for prevalent dementia in NIOC population are under microscope; it would not be unfair to assert that it is the first time that this kind of study is done in the Iranian population. Among the assessed risk factors, only a history of vitamin D, cancer, and chronic anemia showed a significant relationship with an increased chance of dementia.
Consistent with the results of previous studies, the results of this study showed that both less and more than 1 year of vitamin D deficiency have significantly increased the risk of dementia for the individuals. In a 30-year follow-up study, Afzal et al. have shown that vitamin D deficiency was strongly associated with an increased risk of dementia [7]. Similarly, other studies reported similar findings [8, 9]. Some explanations are proposed for the link between dementia and vitamin D deficiency. For example, studies have linked vitamin D deficiency to cerebrovascular pathology, meaning that the deficiency escalates the risk of incident ischemic stroke through the increased number of large vessel infarcts, which in turn, have shown to increase the risk of dementia [5, 10, 11]. Another possible mechanism is that vitamin D receptors in the hippocampus and dentate gyrus are involved in learning and memory.
Similarly, the enzyme responsible for synthesizing 1,25-dihydroxy-vitamin D3 (the active form of vitamin D) is produced in several parts of the brain. The active version of vitamin D is responsible for regulating the expression of nerve growth factor and other factors of neurotrophic expression, as well as the life of neural cells [7]. In laboratory conditions, vitamin D stimulates phagocytic cells, which increases the removal of Beta-amyloid plaques, an indication of Alzheimer’s disease [12]. A previous study showed that the inflammatory process of Alzheimer’s disease is dependent on the pathway receptor-disturbance of vitamin D [12].
It is also established that vitamin D reduces cytotoxicity caused by amyloid and apoptosis in embryonic cortical neurons [13]. The inflammatory process of Alzheimer’s disease is shown to be dependent on the pathway receptor-disturbance of vitamin D [14]. In animal experiments, vitamin D therapy has been shown to improve the decline in memory and learning caused by age [15]. In summary, both vascular and neurodegenerative mechanisms have been proposed for the link between vitamin D deficiency and dementia. The results lend some support to the notion that vitamin D may have neuroprotective roles [16].
A surprising finding of the present study implies that individuals, who had less than 1 year of vitamin D deficiency history (OR=7.50, P<0.001), had a 2.77 point higher chance for dementia compared with those having more than 1 year of vitamin D3 deficiency history (OR=4.73, P<0.001). This finding puts forward the idea that there may be acute and chronic roles for vitamin D deficiency in dementia pathology. Although not mentioned in the previous studies, with the aid of speculation, it can be suggested that acute vitamin D deficiency brings individuals a higher risk of dementia since they do not see the situation as critical and, consequently, do not seek proper treatment; this is the time, when the compensatory mechanisms are less efficient. But, after 1 year, the retaliatory mechanisms start working correctly or individuals start taking medications and lessen their chance of getting dementia. However, further studies are required to be conducted to investigate this phenomenon.
In the present study, we found a significantly higher risk of dementia for individuals with a history of cancer (OR=15.31, P<0.001) compared to those without it. Limited studies in this regard have reported contradictory findings. For example, a meta-analysis of observational studies by Lopez et al. in 2014 showed a weird inverse association between Alzheimer’s disease and cancer; they showed that patients with a history of cancer had 50% less risk of Alzheimer’s disease [17]. Several studies have tried to explain the biological and molecular associations between low predisposition to one type of disease and protection against other diseases [18, 19].
Ming et al. have also reported a significant inverse relationship between hematologic malignancies, lung cancer, and colorectal cancer [20]. However, these associations are rife with partiality since one particular disease may reduce the life of the patients and may not allow them to live longer to experience another or because of the presence of one condition, the other may remain undiscovered. On the other hand, the results of the current study were consistent with a study by Frain et al., where prostate cancer survivors had a moderately higher risk for developing Alzheimer’s disease [21].
Similarly, the results of a cross-sectional study by Heflin et al. suggested that patients with cancer were at a higher risk of cognitive problems in comparison with the ones not experiencing it [22]. A proposed mechanism for the possible link between cancer history and dementia is that the “growth factor progranulin” (PGRN), which has multiple roles, is an extracellular regulatory protein regulating cell division, survival, and inflammation. The levels of PGRN, which are shown to have roles in neurobiology, often increase in cancers. An autosomal mutation in PGRN results in neuronal atrophy in the frontal and temporal lobes and a consequent frontotemporal lobar dementia [23].
The inconsistency observed in our study with previous studies concerning the relationship between cancer and dementia deserves some comments. The majority of the studies mentioned above are conducted in developed countries; the individuals with a cancer diagnosis have access to quality health services or more effective treatment for their condition, which in turn, may result in better health for the individuals and, consequently, less risk of dementia. However, in a less developed country such as Iran, where the health systems have space for more development, it seems that the same biological pathways for cancer increase the risk of dementia for the individuals.
Based on our results, a history of chronic anemia had significantly increased the risk of dementia (OR=5.95, P<0.001), which is consistent with the results of 3 previous studies in this regard. One research showed that in a 3-year follow-up in 1139 older adults, anemia was related to a 2-fold increase in the risk of developing dementia [24]. Another study showed that anemia was linked to a 60% increase in dementia risk for older adults [25]. the third study showed that anemia was related to a significant relative risk of 1.64 for dementia [26].
Even though the mechanisms for the relationship between anemia and dementia have not been clearly understood until now, our results gave some support to this relationship. However, some hypotheses have been developed to outline the mechanisms for this association. The first hypothesis assumes that anemia results in chronic brain hypoxia, which may increase the risk of dementia [24]. Another theory asserts that anemia caused by kidney disease may be related to dementia since, in addition to regulating red blood cell production, erythropoietin receptors in the brain seem to have a protective effect against stroke and hypoxia; also, lower erythropoietin levels may increase the risk of neuronal degeneration [27]. Because of the deficiency of micronutrients such as iron and vitamin B12, anemia may also be associated with cognitive impairment and dementia. The lack of iron may lead to cerebral hypoxia and cognitive decline because it plays a vital role in oxygen transport and storage in the brain [28].
The present study has several strengths, contrary to the other domestic studies on dementia, where dementia diagnoses have mostly been based on questionnaires or the reports of informants; we used the Diagnostic and Statistical Manual of Mental Disorders, fifth edition criteria, as well as neurologist’s examinations, to confirm the presence of dementia. Moreover, this was the first domestic structured case-control study conducted in NIOC retires for assessing the most common medical risk factors for dementia. Finally, because of the relatively big sample size of the present study and the existence of the similar reliable statistics for the elderly population in NIOC compared to the whole country according to Iranian national statistics (www.amar.org.ir), the results of this study can be easily validated by other populations.
Unfortunately, the information collected by the questionnaires was prone to recall bias. What is more, because of the nature of the research, we could not calculate the relative risk for medical and health-related exposures.
5. Conclusion
To recapitulate what we outlined above, the study proposed that vitamin D deficiency, cancer, and chronic anemia increased the risk of dementia in the NIOC retirees. However, the relationship between dementia and risk factors seems to be more complex than what we report here. A complicated interaction among medical, genetic, environmental, and sociodemographic risk factors, as well as the biology of the human body, makes it more urgent to design more casual studies. Finally, since most of the medical and health-related risk factors for dementia are potentially modifiable, preventing, controlling, and monitoring them seem to provide an excellent opportunity for policymakers to better address dementia as a public health priority.
Ethical Considerations
Compliance with ethical guidelines
The proposal of the present study was approved by the University of Social Welfare and Rehabilitation Sciences with the code of ethics: IR.USWR.REC.1396.414
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the support of the research committee of the National Iranian Oil Company. We also thank our participants and their families for their kind cooperation.
References
Full-Text: (1369 Views)
1. Introduction
Today, nearly 50 million people with dementia are living in the world, and it is believed that the figure will have tripled in 30 years [1]. Dementia syndrome is primarily seen in later life and results in memory accompanied by the impairment of other aspects of function [2]. A noticeable rise in life expectancy and global population aging have been deemed to be the leading causes for this phenomenon. Right now, the global annual cost of caring for patients with dementia has surpassed 1 trillion dollars [3]. The intolerable financial burden of care for patients with dementia and the unavailability of an effective treatment for postponing or stopping dementia progression has made it one of the most horrific diseases for the nations and families, while pharmaceutical advances of the illness have not been satisfying [3, 4].
Furthermore, until now, we have not completely comprehended the causes and etiology of the disease, which makes it more urgent to put dementia under the microscope. To cope with common difficulties caused by dementia, taking primary preventive measures such as adjusting modifiable risk factors, including cardiovascular diseases, obesity, diabetes, a sedentary lifestyle, and smoking is shown to be effective [5]. For example, a considerable drop in the rate of Alzheimer’s disease in some countries has been attributed to an effective modulating of the preventable risk factors [6].
In the absence of modifiable risk factors, it becomes highly challenging to explain the possible risk factors responsible for the onset of the disease. Numerous studies have already reported a broad range of risk factors for dementia. However, given the geographical differences in dementia prevalence and incidence, there is no comprehensive domestic study evaluating the possible risk factors for dementia in the Iranian population. Given the considerable differences between the culture and lifestyles of the Iranian community and other countries in the world, validating the findings of other parts of the world in the Iranian population is a highly demanding task. Therefore, this study aimed at providing a synoptic account of the possible medical and health-related risk factors associated with prevalent dementia. The presence of hospital information system, which makes it easy to access medical history information for retirees and staff members of the National Iranian Oil Company (NIOC) and more than 3 decades of the researchers’ work in both operational and managerial positions makes NIOC an ideal place to conduct such studies.
Study population
The subjects were assessed and allocated to one of the following categories:
1) Cases: 180 patients with dementia (using the Diagnostic and Statistical Manual of Mental Disorders, fifth edition criteria, neurologist examination, paraclinical assessments, and brain imaging).
2) Controls: 371 individuals without dementia (The Mini-Mental State Examination cut-off point of >19 for illiterate or low-educated individuals, >27 for individuals having average educational attainment, and >29 for those having an academic educational achievement).
The sample size was calculated, using a power of at least 0.90 and detected an Odds Ratio (OR) of 2; the results revealed that 180 patients were necessary for the case group and 371 for the control group.
Setting
Axiomatically, the inclusion criteria for both the cases and controls were as follow: 1) being 60 years old or older, 2) being the staff member of NIOC with the petroleum industry health insurance coverage, 3) signing the informed consent form to participate in the study.
Selection of patients
All patients from 15 operational areas across the NIOC branches throughout the country referring to the hospitals and health centers of the NIOC or the neurologists working with NIOC in 2017 were included in the present study.
Selection of controls
The controls included proxies or close relatives of the dementia cases, who had no sign of cognitive problems and were visited by the physician or neurologist at the same time as the cases were.
2. Methods
Our strategy for extracting medical risk factors consisted of several phases. In the first phase, we conducted an electronic search on the PubMed and Web of Science databases from their start to January 2017 with the keywords “dementia” OR “Alzheimer” and “risk factor”; relevant primary and reviewed articles were revealed. Next, we scrutinized their abstracts for their relevance. We included articles focusing on fluid biomarkers or genetic factors as well. A factor would be considered a “risk factor” if it had shown a significant link to dementia in studies. If an association was covered by more than one study, we would keep the one with the strongest association with dementia. Articles related to the results other than dementia such as cognitive decline or impairment were excluded. This phase resulted in a list of 38 risk factors for dementia.
In the second phase, a panel of experts, including psychologists, neurologists, geriatrics, and gerontologists reviewed and assessed the items of checklist for the appropriateness and relevance of the items to dementia. The comments of the experts were used to revise and adjust the list. The comments of experts resulted in the omission of some risk factors such as the ones related to cerebrospinal fluids because of their inapplicability.
In the third phase, the list was pretested on a sample of 30 older adults, which was not included in the final sample, to examine its usefulness and to include information about aspects of the patient’s personal life and demographic information. This resulted in the shortening of the list to 21 risk factors and sociodemographic information (Table 2).
In the final phase, the existence of medical risk factors was investigated by referring to the hospital information system of NIOC health centers, which is available since about 2 decades ago and is the archive for frequent routine medical evaluations for the staff and retirees. In this phase, the investigators were blinded to the aims of the research to avoid information bias.
Statistical analyses
The data were analyzed, using SPSS version 22 and STATA version 12. We described the quantitative and qualitative data as mean (standard deviation) and frequency (percentage). We assessed the associations of possible risk factors, using simple and multiple logistic regression. Furthermore, we evaluated the independent effects of the reproductive variables after adjusting for potential confounding variables, including age, marital status, and education using a multiple logistic regression model and the backward selection method. We summarized the results regarding adjusted and unadjusted odds (95% CIs) (Table 2).
3. Results
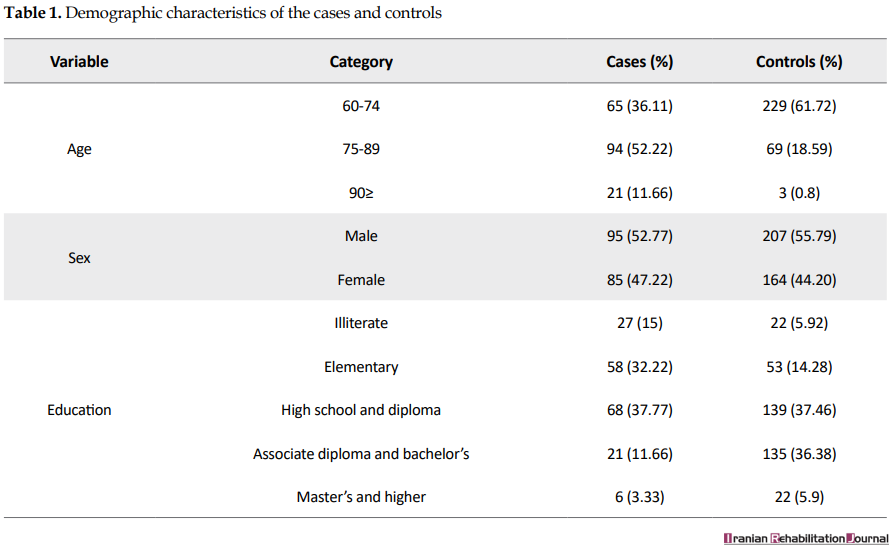
The participation rates of the cases and controls were 95% and 91%, respectively. The majority of the participants were male (55%), married (69%), with a Mean±SD age of 73.14±9.11, and with high school education (38%). Table 1 summarizes the demographic characteristics of the subjects.
Today, nearly 50 million people with dementia are living in the world, and it is believed that the figure will have tripled in 30 years [1]. Dementia syndrome is primarily seen in later life and results in memory accompanied by the impairment of other aspects of function [2]. A noticeable rise in life expectancy and global population aging have been deemed to be the leading causes for this phenomenon. Right now, the global annual cost of caring for patients with dementia has surpassed 1 trillion dollars [3]. The intolerable financial burden of care for patients with dementia and the unavailability of an effective treatment for postponing or stopping dementia progression has made it one of the most horrific diseases for the nations and families, while pharmaceutical advances of the illness have not been satisfying [3, 4].
Furthermore, until now, we have not completely comprehended the causes and etiology of the disease, which makes it more urgent to put dementia under the microscope. To cope with common difficulties caused by dementia, taking primary preventive measures such as adjusting modifiable risk factors, including cardiovascular diseases, obesity, diabetes, a sedentary lifestyle, and smoking is shown to be effective [5]. For example, a considerable drop in the rate of Alzheimer’s disease in some countries has been attributed to an effective modulating of the preventable risk factors [6].
In the absence of modifiable risk factors, it becomes highly challenging to explain the possible risk factors responsible for the onset of the disease. Numerous studies have already reported a broad range of risk factors for dementia. However, given the geographical differences in dementia prevalence and incidence, there is no comprehensive domestic study evaluating the possible risk factors for dementia in the Iranian population. Given the considerable differences between the culture and lifestyles of the Iranian community and other countries in the world, validating the findings of other parts of the world in the Iranian population is a highly demanding task. Therefore, this study aimed at providing a synoptic account of the possible medical and health-related risk factors associated with prevalent dementia. The presence of hospital information system, which makes it easy to access medical history information for retirees and staff members of the National Iranian Oil Company (NIOC) and more than 3 decades of the researchers’ work in both operational and managerial positions makes NIOC an ideal place to conduct such studies.
Study population
The subjects were assessed and allocated to one of the following categories:
1) Cases: 180 patients with dementia (using the Diagnostic and Statistical Manual of Mental Disorders, fifth edition criteria, neurologist examination, paraclinical assessments, and brain imaging).
2) Controls: 371 individuals without dementia (The Mini-Mental State Examination cut-off point of >19 for illiterate or low-educated individuals, >27 for individuals having average educational attainment, and >29 for those having an academic educational achievement).
The sample size was calculated, using a power of at least 0.90 and detected an Odds Ratio (OR) of 2; the results revealed that 180 patients were necessary for the case group and 371 for the control group.
Setting
Axiomatically, the inclusion criteria for both the cases and controls were as follow: 1) being 60 years old or older, 2) being the staff member of NIOC with the petroleum industry health insurance coverage, 3) signing the informed consent form to participate in the study.
Selection of patients
All patients from 15 operational areas across the NIOC branches throughout the country referring to the hospitals and health centers of the NIOC or the neurologists working with NIOC in 2017 were included in the present study.
Selection of controls
The controls included proxies or close relatives of the dementia cases, who had no sign of cognitive problems and were visited by the physician or neurologist at the same time as the cases were.
2. Methods
Our strategy for extracting medical risk factors consisted of several phases. In the first phase, we conducted an electronic search on the PubMed and Web of Science databases from their start to January 2017 with the keywords “dementia” OR “Alzheimer” and “risk factor”; relevant primary and reviewed articles were revealed. Next, we scrutinized their abstracts for their relevance. We included articles focusing on fluid biomarkers or genetic factors as well. A factor would be considered a “risk factor” if it had shown a significant link to dementia in studies. If an association was covered by more than one study, we would keep the one with the strongest association with dementia. Articles related to the results other than dementia such as cognitive decline or impairment were excluded. This phase resulted in a list of 38 risk factors for dementia.
In the second phase, a panel of experts, including psychologists, neurologists, geriatrics, and gerontologists reviewed and assessed the items of checklist for the appropriateness and relevance of the items to dementia. The comments of the experts were used to revise and adjust the list. The comments of experts resulted in the omission of some risk factors such as the ones related to cerebrospinal fluids because of their inapplicability.
In the third phase, the list was pretested on a sample of 30 older adults, which was not included in the final sample, to examine its usefulness and to include information about aspects of the patient’s personal life and demographic information. This resulted in the shortening of the list to 21 risk factors and sociodemographic information (Table 2).
In the final phase, the existence of medical risk factors was investigated by referring to the hospital information system of NIOC health centers, which is available since about 2 decades ago and is the archive for frequent routine medical evaluations for the staff and retirees. In this phase, the investigators were blinded to the aims of the research to avoid information bias.
Statistical analyses
The data were analyzed, using SPSS version 22 and STATA version 12. We described the quantitative and qualitative data as mean (standard deviation) and frequency (percentage). We assessed the associations of possible risk factors, using simple and multiple logistic regression. Furthermore, we evaluated the independent effects of the reproductive variables after adjusting for potential confounding variables, including age, marital status, and education using a multiple logistic regression model and the backward selection method. We summarized the results regarding adjusted and unadjusted odds (95% CIs) (Table 2).
3. Results
The participation rates of the cases and controls were 95% and 91%, respectively. The majority of the participants were male (55%), married (69%), with a Mean±SD age of 73.14±9.11, and with high school education (38%). Table 1 summarizes the demographic characteristics of the subjects.
As it is presented in Table 2, the univariate logistic regression demonstrated that a history of all the medical problems under assessment had significantly increased the chances of dementia. However, because of the high number of variables, we did not include insignificant variables such as homocysteine deficiency, history of high blood lipids, and open-heart operation in Table 2.
Table 3 presents the results of multiple logistic regression after adjusting for age, sex, and education. A history of cancer (OR=15.31, P<0.001) and chronic anemia (OR=5.95, P<0.001) had significantly increased the chances of dementia. Moreover, a history of less (OR=7.50, P<0.001) and more than (OR=4.73, P<0.001) 1 year of vitamin D3 deficiency had also significantly increased the risk of dementia for the individuals.
4. Discussion
In the present study, we evaluated the association between dementia and medical risk factors. Although previous studies have reported different risk factors for dementia, but as far as we know, it is the first time that the risk factors for prevalent dementia in NIOC population are under microscope; it would not be unfair to assert that it is the first time that this kind of study is done in the Iranian population. Among the assessed risk factors, only a history of vitamin D, cancer, and chronic anemia showed a significant relationship with an increased chance of dementia.
Consistent with the results of previous studies, the results of this study showed that both less and more than 1 year of vitamin D deficiency have significantly increased the risk of dementia for the individuals. In a 30-year follow-up study, Afzal et al. have shown that vitamin D deficiency was strongly associated with an increased risk of dementia [7]. Similarly, other studies reported similar findings [8, 9]. Some explanations are proposed for the link between dementia and vitamin D deficiency. For example, studies have linked vitamin D deficiency to cerebrovascular pathology, meaning that the deficiency escalates the risk of incident ischemic stroke through the increased number of large vessel infarcts, which in turn, have shown to increase the risk of dementia [5, 10, 11]. Another possible mechanism is that vitamin D receptors in the hippocampus and dentate gyrus are involved in learning and memory.
Similarly, the enzyme responsible for synthesizing 1,25-dihydroxy-vitamin D3 (the active form of vitamin D) is produced in several parts of the brain. The active version of vitamin D is responsible for regulating the expression of nerve growth factor and other factors of neurotrophic expression, as well as the life of neural cells [7]. In laboratory conditions, vitamin D stimulates phagocytic cells, which increases the removal of Beta-amyloid plaques, an indication of Alzheimer’s disease [12]. A previous study showed that the inflammatory process of Alzheimer’s disease is dependent on the pathway receptor-disturbance of vitamin D [12].
It is also established that vitamin D reduces cytotoxicity caused by amyloid and apoptosis in embryonic cortical neurons [13]. The inflammatory process of Alzheimer’s disease is shown to be dependent on the pathway receptor-disturbance of vitamin D [14]. In animal experiments, vitamin D therapy has been shown to improve the decline in memory and learning caused by age [15]. In summary, both vascular and neurodegenerative mechanisms have been proposed for the link between vitamin D deficiency and dementia. The results lend some support to the notion that vitamin D may have neuroprotective roles [16].
A surprising finding of the present study implies that individuals, who had less than 1 year of vitamin D deficiency history (OR=7.50, P<0.001), had a 2.77 point higher chance for dementia compared with those having more than 1 year of vitamin D3 deficiency history (OR=4.73, P<0.001). This finding puts forward the idea that there may be acute and chronic roles for vitamin D deficiency in dementia pathology. Although not mentioned in the previous studies, with the aid of speculation, it can be suggested that acute vitamin D deficiency brings individuals a higher risk of dementia since they do not see the situation as critical and, consequently, do not seek proper treatment; this is the time, when the compensatory mechanisms are less efficient. But, after 1 year, the retaliatory mechanisms start working correctly or individuals start taking medications and lessen their chance of getting dementia. However, further studies are required to be conducted to investigate this phenomenon.
In the present study, we found a significantly higher risk of dementia for individuals with a history of cancer (OR=15.31, P<0.001) compared to those without it. Limited studies in this regard have reported contradictory findings. For example, a meta-analysis of observational studies by Lopez et al. in 2014 showed a weird inverse association between Alzheimer’s disease and cancer; they showed that patients with a history of cancer had 50% less risk of Alzheimer’s disease [17]. Several studies have tried to explain the biological and molecular associations between low predisposition to one type of disease and protection against other diseases [18, 19].
Ming et al. have also reported a significant inverse relationship between hematologic malignancies, lung cancer, and colorectal cancer [20]. However, these associations are rife with partiality since one particular disease may reduce the life of the patients and may not allow them to live longer to experience another or because of the presence of one condition, the other may remain undiscovered. On the other hand, the results of the current study were consistent with a study by Frain et al., where prostate cancer survivors had a moderately higher risk for developing Alzheimer’s disease [21].
Similarly, the results of a cross-sectional study by Heflin et al. suggested that patients with cancer were at a higher risk of cognitive problems in comparison with the ones not experiencing it [22]. A proposed mechanism for the possible link between cancer history and dementia is that the “growth factor progranulin” (PGRN), which has multiple roles, is an extracellular regulatory protein regulating cell division, survival, and inflammation. The levels of PGRN, which are shown to have roles in neurobiology, often increase in cancers. An autosomal mutation in PGRN results in neuronal atrophy in the frontal and temporal lobes and a consequent frontotemporal lobar dementia [23].
The inconsistency observed in our study with previous studies concerning the relationship between cancer and dementia deserves some comments. The majority of the studies mentioned above are conducted in developed countries; the individuals with a cancer diagnosis have access to quality health services or more effective treatment for their condition, which in turn, may result in better health for the individuals and, consequently, less risk of dementia. However, in a less developed country such as Iran, where the health systems have space for more development, it seems that the same biological pathways for cancer increase the risk of dementia for the individuals.
Based on our results, a history of chronic anemia had significantly increased the risk of dementia (OR=5.95, P<0.001), which is consistent with the results of 3 previous studies in this regard. One research showed that in a 3-year follow-up in 1139 older adults, anemia was related to a 2-fold increase in the risk of developing dementia [24]. Another study showed that anemia was linked to a 60% increase in dementia risk for older adults [25]. the third study showed that anemia was related to a significant relative risk of 1.64 for dementia [26].
Even though the mechanisms for the relationship between anemia and dementia have not been clearly understood until now, our results gave some support to this relationship. However, some hypotheses have been developed to outline the mechanisms for this association. The first hypothesis assumes that anemia results in chronic brain hypoxia, which may increase the risk of dementia [24]. Another theory asserts that anemia caused by kidney disease may be related to dementia since, in addition to regulating red blood cell production, erythropoietin receptors in the brain seem to have a protective effect against stroke and hypoxia; also, lower erythropoietin levels may increase the risk of neuronal degeneration [27]. Because of the deficiency of micronutrients such as iron and vitamin B12, anemia may also be associated with cognitive impairment and dementia. The lack of iron may lead to cerebral hypoxia and cognitive decline because it plays a vital role in oxygen transport and storage in the brain [28].
The present study has several strengths, contrary to the other domestic studies on dementia, where dementia diagnoses have mostly been based on questionnaires or the reports of informants; we used the Diagnostic and Statistical Manual of Mental Disorders, fifth edition criteria, as well as neurologist’s examinations, to confirm the presence of dementia. Moreover, this was the first domestic structured case-control study conducted in NIOC retires for assessing the most common medical risk factors for dementia. Finally, because of the relatively big sample size of the present study and the existence of the similar reliable statistics for the elderly population in NIOC compared to the whole country according to Iranian national statistics (www.amar.org.ir), the results of this study can be easily validated by other populations.
Unfortunately, the information collected by the questionnaires was prone to recall bias. What is more, because of the nature of the research, we could not calculate the relative risk for medical and health-related exposures.
5. Conclusion
To recapitulate what we outlined above, the study proposed that vitamin D deficiency, cancer, and chronic anemia increased the risk of dementia in the NIOC retirees. However, the relationship between dementia and risk factors seems to be more complex than what we report here. A complicated interaction among medical, genetic, environmental, and sociodemographic risk factors, as well as the biology of the human body, makes it more urgent to design more casual studies. Finally, since most of the medical and health-related risk factors for dementia are potentially modifiable, preventing, controlling, and monitoring them seem to provide an excellent opportunity for policymakers to better address dementia as a public health priority.
Ethical Considerations
Compliance with ethical guidelines
The proposal of the present study was approved by the University of Social Welfare and Rehabilitation Sciences with the code of ethics: IR.USWR.REC.1396.414
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors appreciate the support of the research committee of the National Iranian Oil Company. We also thank our participants and their families for their kind cooperation.
References
- International Alzheimer’s Disease International (AsD). World Alzheimer report 2010: The global economic impact of dementia. London: Alzheimer’s Disease International; 2010.
- Fillit HM, Rockwood K, Young JB. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology E-Book. Edinburgh: Elsevier Health Sciences; 2016.
- Gauthier S, Albert M, Fox N, Goedert M, Kivipelto M, Mestre-Ferrandiz J, et al. Why has therapy development for dementia failed in the last two decades? Alzheimer’s & Dementia. 2016; 12(1):60-4. [DOI:10.1016/j.jalz.2015.12.003] [PMID]
- Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. Epidemiology of dementias and Alzheimer’s disease. Archives of Medical Research. 2012; 43(8):600-8. [DOI:10.1016/j.arcmed.2012.11.003] [PMID]
- Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JP, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimer’s & Dementia. 2017; 13(4):406-18. [DOI:10.1016/j.jalz.2016.07.152] [PMID]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. The Lancet Neurology. 2014; 13(8):788-94. [DOI:10.1016/S1474-4422(14)70136-X]
- Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer’s disease and vascular dementia. Alzheimer’s & Dementia. 2014; 10(3):296-302. [DOI:10.1016/j.jalz.2013.05.1765] [PMID]
- Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014; 83(10):920-8. [DOI:10.1212/WNL.0000000000000755] [PMID] [PMCID]
- Annweiler C, Rolland Y, Schott A, Blain H, Vellas B, Beauchet O. Serum vitamin D deficiency as a predictor of incident non-Alzheimer dementias: A 7-year longitudinal study. Dementia and Geriatric Cognitive Disorders. 2011; 32(4):273-8. [DOI:10.1159/000334944] [PMID]
- Brøndum‐Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25‐hydroxyvitamin D, and symptomatic ischemic stroke: An original study and meta‐analysis. Annals of Neurology. 2013; 73(1):38-47. [DOI:10.1002/ana.23738] [PMID]
- Tatemichi T, Paik M, Bagiella E, Desmond D, Stern Y, Sano M, et al. Risk of dementia after stroke in a hospitalized cohort: Results of a longitudinal study. Neurology. 1994; 44(10):1885. [DOI:10.1212/WNL.44.10.1885] [PMID]
- Mizwicki MT, Menegaz D, Zhang J, Barrientos-Durán A, Tse S, Cashman JR, et al. Genomic and nongenomic signaling induced by 1α, 25 (OH) 2-vitamin D 3 promotes the recovery of amyloid-β phagocytosis by Alzheimer’s disease macrophages. Journal of Alzheimer’s Disease. 2012; 29(1):51-62. [DOI:10.3233/JAD-2012-110560] [PMID]
- Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by amyloid-β and preventing the amyloid-β induced alterations by vitamin D in cortical neurons. Journal of Alzheimer’s Disease. 2011; 23(2):207-19. [DOI:10.3233/JAD-2010-101377] [PMID]
- Dursun E, Gezen-Ak D, Yilmazer S. A new mechanism for amyloid-β induction of iNOS: vitamin D-VDR pathway disruption. Journal of Alzheimer’s Disease. 2013; 36(3):459-74. [DOI:10.3233/JAD-130416] [PMID]
- Briones TL, Darwish H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. Journal of Neuroinflammation. 2012; 9(1):244. [DOI:10.1186/1742-2094-9-244] [PMID] [PMCID]
- Dickens AP, Lang IA, Langa KM, Kos K, Llewellyn DJ. Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs. 2011; 25(8):629-39. [DOI:10.2165/11593080-000000000-00000] [PMID] [PMCID]
- Catalá-López F, Suárez-Pinilla M, Suárez-Pinilla P, Valderas JM, Gómez-Beneyto M, Martinez S, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: A meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychotherapy and Psychosomatics. 2014; 83(2):89-105. [DOI:10.1159/000356498] [PMID]
- Behrens MI, Lendon C, Roe CM. A common biological mechanism in cancer and Alzheimer’s disease? Current Alzheimer Research. 2009; 6(3):196-204. [DOI:10.2174/156720509788486608] [PMID] [PMCID]
- Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A. Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genetics. 2014; 10(2):e1004173. [DOI:10.1371/journal.pgen.1004173] [PMID] [PMCID]
- Ou SM, Lee YJ, Hu YW, Liu CJ, Chen TJ, Fuh JL, et al. Does Alzheimer’s disease protect against cancers? A nationwide Population-based Study. Neuroepidemiology. 2013; 40(1):42-9. [DOI:10.1159/000341411] [PMID]
- Frain L, Swanson D, Cho K, Gagnon D, Lu KP, Betensky RA, et al. Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimer’s & Dementia. 2017; 13(12):1364-70. [DOI:10.1016/j.jalz.2017.04.012] [PMID] [PMCID]
- Heflin LH, Meyerowitz BE, Hall P, Lichtenstein P, Johansson B, Pedersen NL, et al. Cancer as a risk factor for long-term cognitive deficits and dementia. Journal of the National Cancer Institute. 2005; 97(11):854-6. [DOI:10.1093/jnci/dji137] [PMID]
- Bateman A, Bennett HP. The granulin gene family: From cancer to dementia. Bioessays. 2009; 31(11):1245-54. [DOI:10.1002/bies.200900086] [PMID]
- Atti AR, Palmer K, Volpato S, Zuliani G, Winblad B, Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiology of Aging. 2006; 27(2):278-84. [DOI:10.1016/j.neurobiolaging.2005.02.007] [PMID]
- Shah R, Buchman A, Wilson R, Leurgans S, Bennett D. Hemoglobin level in older persons and incident Alzheimer disease Prospective cohort analysis. Neurology. 2011: 77(3):219-26.
- Hong CH, Falvey C, Harris TB, Simonsick EM, Satterfield S, Ferrucci L, et al. Anemia and risk of dementia in older adults Findings from the Health ABC study. Neurology. 2013; 81(6):528-33. [DOI:10.1212/WNL.0b013e31829e701d] [PMID] [PMCID]
- Assaraf MI, Diaz Z, Liberman A, Miller Jr WH, Arvanitakis Z, Li Y, et al. Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. Journal of Neuropathology and Experimental Neurology. 2007; 66(5):389-98. [DOI:10.1097/nen.0b013e3180517b28] [PMID]
- Yavuz BB, Cankurtaran M, Haznedaroglu I, Halil M, Ulger Z, Altun B, et al. Iron deficiency can cause cognitive impairment in geriatric patients. The Journal of Nutrition, Health & Aging. 2012; 16(3):220-4. [DOI:10.1007/s12603-011-0351-7]
Article type: Original Research Articles |
Subject:
Aging Studies
Received: 2019/01/25 | Accepted: 2019/06/1 | Published: 2019/12/29
Received: 2019/01/25 | Accepted: 2019/06/1 | Published: 2019/12/29
Send email to the article author