Volume 21, Issue 4 (December 2023)
Iranian Rehabilitation Journal 2023, 21(4): 743-750 |
Back to browse issues page
Ethics code: approval No: P.T.REC/012/003833
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
AL-Nemr A. Correlation Between Body Mass Index, Selectivity, and Functional Independence in Children With Cerebral Palsy. Iranian Rehabilitation Journal 2023; 21 (4) :743-750
URL: http://irj.uswr.ac.ir/article-1-1818-en.html
URL: http://irj.uswr.ac.ir/article-1-1818-en.html
Department of Physical Therapy for Pediatrics, Faculty of Physical Therapy, Cairo University, Giza, Egypt.
Full-Text [PDF 520 kb]
(212 Downloads)
| Abstract (HTML) (1346 Views)
Full-Text: (123 Views)
Introduction
Cerebral palsy (CP) is a disorder classified clinically according to the predominant motor syndrome and characterized by abnormal posture, tone, and movement.[1] The prevalence of CP in high-income countries is about 1.6/1000 live births, while low-income countries showed marked higher birth prevalence [2]. Also, there is a higher prevalence of 60 to 150 per 1000 live births among preterm infants [3]. The most prevalent type of CP is spastic diplegia which is commonly associated with prematurity [4]. Children with spastic CP suffer from multiple neurological abnormalities including muscle weakness, spasticity, and abnormal movement pattern [1]. Abnormal movement pattern occurs in children with spastic CP due to coordination problems which are caused by extensor or flexor synergies that interfere with isolated joint movement [5].
Selective voluntary motor control (SVMC) is an integral part of normal human motion and can independently control joint movement. Reduced SVMC is defined as a disturbance of the capability of isolating the muscle activation in selected patterns due to voluntary movement or posture demands [6] which means that a child with impaired SVMC does not have the expected activation pattern which is present in healthy children, either due to lack or excessive muscle activity [7]. This is one of the neuromuscular disorders associated with spastic CP and commonly coexists with muscle weakness and spasticity. All of the previously mentioned motor disorders are related to the damage of the corticospinal tract and other descending motor pathways, resulting in interruption of the descending excitability and inhibitory signals leading to dysfunction [8, 9].
Children with CP have less energy consumption, and limited participation in physical activities compatible with their age compared to their healthy peers. Thus, the risk of obesity in these children is higher than in healthy ones [10]. The prevalence of obesity in children with CP who are ambulant has increased from 7.7% to 16.5% in the last decade which affect negatively the general health of these children [11]. Through the analysis of body mass index (BMI) and waist-hip ratio, Bansal and his colleagues suggest that children with CP have a high rate of being overweight and are at risk of being obese, particularly central obesity [12]. Fat mass can be assessed by several tools such as hydrostatic weighing and dual-energy x-ray absorptiometry which are valid tools for measuring obesity. However, these tools have limited usage and are more invasive than BMI. So, BMI is widely recommended by many institutions as the World Health Organization (WHO) and the World Obesity Federation [13, 14].
The risk of becoming overweight or obese increases throughout the range of dysfunction in children who have mild to moderate motor disabilities [15]. Obesity is of particular concern to children with CP generally because of the long-term health risks associated with obesity and because of the potentially increased impairment of functional mobility associated with obesity [16]. Dixon magnetic resonance images showed a lower intramuscular fat ratio in normal children compared to children with spastic CP who can ambulate [17]. Also, bioelectrical impedance analysis was used to estimate fat-free mass in preschool children with CP, and altered body composition was detected in these children across functional capacities [18].
Former studies have shown the relationship between lower limb SVMC and gait in children with CP [19, 20], and between health-related quality of life and BMI [21], but no available studies are detected regarding the relationship between BMI, isolated motor control, and functional independence in children with diplegic CP. The purpose of this study was to detect the relationship between BMI, SVMC, and functional independence in these children.
Materials and Methods
Study design and setting
This is an observational correlational study held at the Faculty of Physical Therapy, Cairo University between January 2022 and August 2022, carried out in the Outpatient Clinic.
Sample size
G*Power statistical software, version 3.1 (Germany) was chosen for estimating the sample size before the study using effect size=0.3, β=0.2, and α=0.05 which were calculated from a pilot study, showed a total sample size of 84 children for this study. Ninety children were enrolled for prospective dropouts.
Participant recruitment and selection criteria
Initially, 90 children were enrolled and six children were excluded: Two children had lower limb surgical operations, and four children quit before the assessment termination. A total of 84 children with spastic diplegic CP, (32 boys and 52 girls) get involved in this study. The participants were randomly selected from the Outpatient Clinic at the Faculty of Physical Therapy, Cairo University.
Children diagnosed as spastic diplegic CP, whose chronological age ranges from 6 up to 9 years, with spasticity levels 1+ and 2 in the affected lower limbs as specified by the modified Ashworth scale (MAS), and levels II and III of the gross motor functional classification system expanded and revised (GMFCS E&R), who were able to follow the given instructions during the assessment were included in this study. Children who had contractures of the lower limb joints were injected with Botulinum toxin or any orthopedic surgery of the lower extremities in the last 6 months before the study, or who had epilepsy, were excluded from the study. Table 1 shows the characteristics of the participating children.

Testing procedures
Anthropometric measurements
A calibrated digital scale was used to measure children’s weight and Harpenden Stadiometer to measure their height. BMI was calculated as weight (kg)/height (m2). Growth charts for children and adolescents with ages ranging from 2–20 years were used to calculate the percentile scores of BMI, which consider the child’s age and sex. BMI was assigned, using these charts, to one of four categories: Underweight, healthy, overweight, or obese [22].
Selective control assessment of the lower extremity (SCALE)
The selective control assessment of the lower extremity is a reliable and valid scale used for evaluating SVMC of the toes, subtalar, ankle, knee, and hip joints in children with spastic CP [23]. The isolated movements are evaluated bilaterally. Scores of normal “2”, impaired “1”, or unable “0” were given for each joint, 10 points for each limb, with 20 points as a total maximum score. Children were instructed to do specific isolated movements - reported in the SCALE - at each joint using a 3-second verbal count [23].
Pediatric functional independence measure (Wee-FIM)
The pediatric functional independence measure (Wee-FIM) is a reliable and valid scale used for evaluating the daily life activities of children with CP [24]. The reliability and feasibility of this scale were reported in Egyptian children, although with different diagnoses [25]. Evaluation can be supplemented by observation of the child and an interview with the caregiver, with a 20-minute administration time. This scale assesses the physical and cognitive ability of the child. It includes 6 domains of function which were evaluated in this study; four of these are physical and two are cognitive. Items are scored with a 7-point ordinal scale, with 7 meaning complete independence and 1 meaning total dependency. Caregiver assistance is needed for scores of 1 to 5. Higher scores indicate more independence with possible scores ranging from 18 to 126 [26].
The gross motor function classification system expanded and revised (GMFCS E&R)
The gross motor function classification system is a valid and reliable classification that differentiates children with CP depending on their gross motor abilities and limitations [27]. It also showed high reliability for its Arabic version [28]. It contains 5 levels [29]. The distinction between different levels ranges from level I, the highest gross motor function up to level V, the lowest level, based on the need for assistive technology and functional abilities rather than the quality of movement [30]. Nearly 98% of children with diplegia are categorized into Level I, II, or III [31]. Since children with Level I of GMFCS were more likely to have a healthy weight [32], only levels II and III were included in this study.
Modified Ashworth scale (MAS)
The modified Ashworth scale is a widely accepted clinical tool that is used to measure spasticity. It is a 6-point ordinal scale with a grade score of 0, 1, 1+, 2, 3, and 4, where a higher grade score indicates increased spasticity [33]. According to the MAS scoring system, the selected children were graded 1+and 2 which are the most available grades in the outpatient clinic during this study.
Statistical analysis
The relationship between BMI, SVMC, and the level of functional independence in children with diplegic CP was assessed using correlation analysis. The statistical package for the social sciences program, version 25 (IBM SPSS, Chicago, IL, USA) was used for analyses. Descriptive statistics were expressed in numerical (n) and percent (%) formats, and mean±standard deviation. Spearman’s correlation coefficient was selected to detect the relationships due to the ordinal nature of the data. A correlation was considered significant at a p<0.05.
Results
In this study, 84 children with spastic diplegic CP were selected. Table 1 displays the general characteristics of the children participating in the study. There was a positive, strong, and significant correlation between MAS and BMI (rs=+0.677, P<0.001); between WEE FIM total score and SCALE (rs=+0.718, P<0.001); between the physical dimension of WEE FIM and SCALE (rs=+0.843, P<0.001); and between the cognitive dimension of WEE FIM and SCALE (rs=+0.904, P<0.001), while there was a negative, strong, and significant correlation between MAS and WEE FIM total score (rs=-0.669, P<0.001), between MAS and the physical dimension of WEE FIM (rs=-0.712, P<0.001), between MAS and the cognitive dimension of WEE FIM, (rs=-0.793, P<0.001), between MAS and SCALE (rs=-0.830, P<0.001), between the cognitive dimension of WEE FIM and GMFCS(rs=-0.660, P<0.001), between SCALE and GMFCS (rs=-0.707, P<0.001), and between WEE FIM total score and BMI (rs=-0.607, P<0.001) as presented in Table 2.
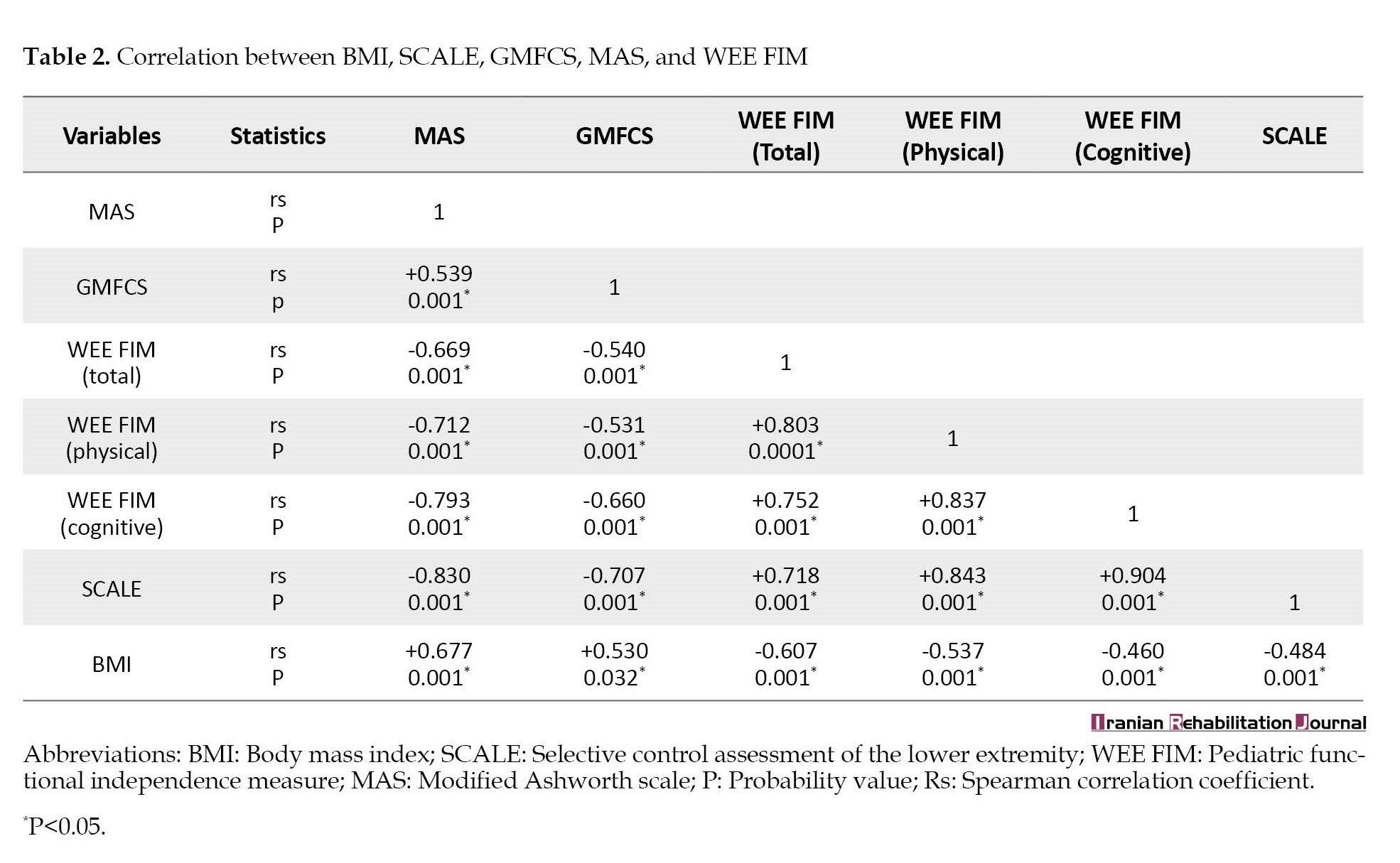
In addition, there was a positive, moderate, and significant correlation between MAS and GMFCS (rs=+0.539, P<0.001) and between BMI and GMFCS (rs=+0.53, P<0.032). There was a negative, moderate, and significant correlation between GMFCS and WEE FIM total score(rs=-0.540, P<0.001), between GMFCS and the physical dimension of WEE FIM (rs=-0.531, P<0.001), between BMI and SCALE(rs=-0.484, P<0.001), between BMI and the physical dimension of WEEFIM (rs=-0.537, P<0.001), and between BMI and the cognitive dimension of WEE FIM (rs=-0.460, P<0.001), as presented in Table 2.
Regarding the WEE FIM scale, there was a positive, strong, and significant correlation between the WEE FIM total score with its physical and cognitive dimensions (rs=+0.803, P<0.001), (rs=+0.752, P=0.001) respectively, and between the physical and cognitive dimensions of WEE FIM scale (rs=+0.837, P<0.001), as presented in Table 2.
Discussion
Children with spastic diplegia have disabilities of the lower extremities resulting in dependency on their functional daily activities. Spasticity, muscle weakness, and activity limitations contribute to a lack of isolated motor control, limitations in their daily living activities, and obesity in some children [34, 35]. The findings of this study showed a significant correlation between BMI, SVMC, functional independence, gross motor function, and spasticity in the participating children.
Many studies suggest different mechanisms by which children with CP are liable to become obese. One such mechanism is that children with CP often are premature babies which is associated with obesity [36]. In addition, children with CP face unique difficulties in the issues related to physical activity and functional ability. So, it is not surprising that children with CP have a higher prevalence of overweight and obesity compared to their normal peers, especially the ones who can walk [21, 37]. In parallel with these studies, this study showed a significant negative correlation between functional independence and BMI.
The body composition of CP is affected by dietary components and also non-nutritional aspects such as impaired mobility, atypical movement patterns, motor impairment severity, and type of CP, that affect obesity. Karatekin et al. [38] mentioned that normal children had a lower obesity rate when compared to ambulant children with CP. In addition, they found that gross motor function was significantly associated with BMI and weight.
Oftedal et al. [16] added that children with GMFCS levels II–V have a higher body fat percentage than those children with GMFCS level I, which is consistent with the positive significant correlation between GMFCS E&R and BMI in this study. So, it is important to measure and follow BMI during the evaluation and rehabilitation of children with diplegic CP when the goal is to improve their functional independence and gross motor functions.
A higher BMI is associated with diminished executive functions and a thinner prefrontal cortex in children [39]. Meo et al. [40] observed significant impairments in cognitive functions in markedly obese students. This comes in agreement with the results of this study which reported a negative significant correlation between BMI and the cognitive dimension of WEE FIM.
The response to physical activity in children with CP may vary by the motor impairment degree and the type of the disorder. So, in contrast to the results of this study which showed a positive significant correlation between GMFCS E&R and BMI, Arshad and Arora [41] showed a negative correlation. This can be illustrated by including all five levels of GMFCS E&R in their study, not only levels II and III.
Being active is more difficult for children with CP who have more severe motor affection than children who have mild impairment. They may have cognitive, perceptive, behavioral, or communication disorders in addition to disorders of muscle tone and strength, that negatively affect their functional ability in different ways [42]. This supports the results of the current study which found a negative significant correlation between gross motor function level with both functional ability and SVMC. Arruda et al. [42] added that in addition to the reduction in muscle strength and high muscle tone, other factors can likely explain more affected functional ability among children with greater motor impairment such as skeletal muscle deformities and more frequent use of medications. This can illustrate the negative significant correlation between functional independence with both spasticity and gross motor function level in this study.
Reduced selective motor control and increased resistance to joint movement are important factors that influence gross motor function. Noble et al. [8] found a positive significant correlation between gross motor function and selectivity (r=0.901), and a negative significant correlation between gross motor function and MAS (r=-0.691), which are in agreement with the results of this study.
Children with CP often have low or normal muscle tone at the beginning of their life which increases in most children up to 5 years of age [43]. For this reason, children’s age in this study was selected to start from 6 years.
Selective motor control disorders are affected by brain damage and are difficult to improve. Thus, SVMC is an important factor that affects motor function in children with CP. In this study, there was a negative strong correlation between SCALE scores and levels of gross motor function which is similar to findings of previous studies of SVMC in children with CP which reported that SCALE is strongly and significantly correlated with GMFM scale in children with bilateral CP [44, 45].
Conclusion
This study shows a significant relationship between BMI, SVMC, and functional independence in children with spastic diplegia. These results could have a remarkable future effect in educational, experimental, and clinical settings. This study provides evidence that BMI and SVMC impairments should be included while studying and clinically evaluating children with diplegic CP. Therapeutic intervention of SVMC should be carefully considered when planning a program to increase the functional independence of children with spastic diplegia. Also, regular height and weight measurement is an important factor in the rehabilitation programs that aim to increase functional abilities in these children.
Limitations
The study included only children with diplegic CP, which may restrict the findings’ generalizability to other types of CP. The study included only children with GMFCS E&R levels II and III, excluding severely disabled children which may limit the findings’ generalizability to other levels of GMFCS E&R. The study used MAS to assess spasticity, which has been questioned for its validity. More advanced measurements of spasticity, such as electromyography, may provide more accurate assessments of spasticity. Finally, the study did not account for the effects of medication use on the study outcomes, which may have confounded the results.
Ethical Considerations
Compliance with ethical guidelines
Children were only allowed to be included in this study after having a signed written consent form from their parents. The clinical trial registration number of this study is NCT05428865. The research has followed the tenets of the Declaration of Helsinki and has been approved by the Faculty of Physical Therapy; Cairo University (No.: P.T.REC/012/003833).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgments
The author would like to thank all the children who participated in the study and their parents.
References
Cerebral palsy (CP) is a disorder classified clinically according to the predominant motor syndrome and characterized by abnormal posture, tone, and movement.[1] The prevalence of CP in high-income countries is about 1.6/1000 live births, while low-income countries showed marked higher birth prevalence [2]. Also, there is a higher prevalence of 60 to 150 per 1000 live births among preterm infants [3]. The most prevalent type of CP is spastic diplegia which is commonly associated with prematurity [4]. Children with spastic CP suffer from multiple neurological abnormalities including muscle weakness, spasticity, and abnormal movement pattern [1]. Abnormal movement pattern occurs in children with spastic CP due to coordination problems which are caused by extensor or flexor synergies that interfere with isolated joint movement [5].
Selective voluntary motor control (SVMC) is an integral part of normal human motion and can independently control joint movement. Reduced SVMC is defined as a disturbance of the capability of isolating the muscle activation in selected patterns due to voluntary movement or posture demands [6] which means that a child with impaired SVMC does not have the expected activation pattern which is present in healthy children, either due to lack or excessive muscle activity [7]. This is one of the neuromuscular disorders associated with spastic CP and commonly coexists with muscle weakness and spasticity. All of the previously mentioned motor disorders are related to the damage of the corticospinal tract and other descending motor pathways, resulting in interruption of the descending excitability and inhibitory signals leading to dysfunction [8, 9].
Children with CP have less energy consumption, and limited participation in physical activities compatible with their age compared to their healthy peers. Thus, the risk of obesity in these children is higher than in healthy ones [10]. The prevalence of obesity in children with CP who are ambulant has increased from 7.7% to 16.5% in the last decade which affect negatively the general health of these children [11]. Through the analysis of body mass index (BMI) and waist-hip ratio, Bansal and his colleagues suggest that children with CP have a high rate of being overweight and are at risk of being obese, particularly central obesity [12]. Fat mass can be assessed by several tools such as hydrostatic weighing and dual-energy x-ray absorptiometry which are valid tools for measuring obesity. However, these tools have limited usage and are more invasive than BMI. So, BMI is widely recommended by many institutions as the World Health Organization (WHO) and the World Obesity Federation [13, 14].
The risk of becoming overweight or obese increases throughout the range of dysfunction in children who have mild to moderate motor disabilities [15]. Obesity is of particular concern to children with CP generally because of the long-term health risks associated with obesity and because of the potentially increased impairment of functional mobility associated with obesity [16]. Dixon magnetic resonance images showed a lower intramuscular fat ratio in normal children compared to children with spastic CP who can ambulate [17]. Also, bioelectrical impedance analysis was used to estimate fat-free mass in preschool children with CP, and altered body composition was detected in these children across functional capacities [18].
Former studies have shown the relationship between lower limb SVMC and gait in children with CP [19, 20], and between health-related quality of life and BMI [21], but no available studies are detected regarding the relationship between BMI, isolated motor control, and functional independence in children with diplegic CP. The purpose of this study was to detect the relationship between BMI, SVMC, and functional independence in these children.
Materials and Methods
Study design and setting
This is an observational correlational study held at the Faculty of Physical Therapy, Cairo University between January 2022 and August 2022, carried out in the Outpatient Clinic.
Sample size
G*Power statistical software, version 3.1 (Germany) was chosen for estimating the sample size before the study using effect size=0.3, β=0.2, and α=0.05 which were calculated from a pilot study, showed a total sample size of 84 children for this study. Ninety children were enrolled for prospective dropouts.
Participant recruitment and selection criteria
Initially, 90 children were enrolled and six children were excluded: Two children had lower limb surgical operations, and four children quit before the assessment termination. A total of 84 children with spastic diplegic CP, (32 boys and 52 girls) get involved in this study. The participants were randomly selected from the Outpatient Clinic at the Faculty of Physical Therapy, Cairo University.
Children diagnosed as spastic diplegic CP, whose chronological age ranges from 6 up to 9 years, with spasticity levels 1+ and 2 in the affected lower limbs as specified by the modified Ashworth scale (MAS), and levels II and III of the gross motor functional classification system expanded and revised (GMFCS E&R), who were able to follow the given instructions during the assessment were included in this study. Children who had contractures of the lower limb joints were injected with Botulinum toxin or any orthopedic surgery of the lower extremities in the last 6 months before the study, or who had epilepsy, were excluded from the study. Table 1 shows the characteristics of the participating children.

Testing procedures
Anthropometric measurements
A calibrated digital scale was used to measure children’s weight and Harpenden Stadiometer to measure their height. BMI was calculated as weight (kg)/height (m2). Growth charts for children and adolescents with ages ranging from 2–20 years were used to calculate the percentile scores of BMI, which consider the child’s age and sex. BMI was assigned, using these charts, to one of four categories: Underweight, healthy, overweight, or obese [22].
Selective control assessment of the lower extremity (SCALE)
The selective control assessment of the lower extremity is a reliable and valid scale used for evaluating SVMC of the toes, subtalar, ankle, knee, and hip joints in children with spastic CP [23]. The isolated movements are evaluated bilaterally. Scores of normal “2”, impaired “1”, or unable “0” were given for each joint, 10 points for each limb, with 20 points as a total maximum score. Children were instructed to do specific isolated movements - reported in the SCALE - at each joint using a 3-second verbal count [23].
Pediatric functional independence measure (Wee-FIM)
The pediatric functional independence measure (Wee-FIM) is a reliable and valid scale used for evaluating the daily life activities of children with CP [24]. The reliability and feasibility of this scale were reported in Egyptian children, although with different diagnoses [25]. Evaluation can be supplemented by observation of the child and an interview with the caregiver, with a 20-minute administration time. This scale assesses the physical and cognitive ability of the child. It includes 6 domains of function which were evaluated in this study; four of these are physical and two are cognitive. Items are scored with a 7-point ordinal scale, with 7 meaning complete independence and 1 meaning total dependency. Caregiver assistance is needed for scores of 1 to 5. Higher scores indicate more independence with possible scores ranging from 18 to 126 [26].
The gross motor function classification system expanded and revised (GMFCS E&R)
The gross motor function classification system is a valid and reliable classification that differentiates children with CP depending on their gross motor abilities and limitations [27]. It also showed high reliability for its Arabic version [28]. It contains 5 levels [29]. The distinction between different levels ranges from level I, the highest gross motor function up to level V, the lowest level, based on the need for assistive technology and functional abilities rather than the quality of movement [30]. Nearly 98% of children with diplegia are categorized into Level I, II, or III [31]. Since children with Level I of GMFCS were more likely to have a healthy weight [32], only levels II and III were included in this study.
Modified Ashworth scale (MAS)
The modified Ashworth scale is a widely accepted clinical tool that is used to measure spasticity. It is a 6-point ordinal scale with a grade score of 0, 1, 1+, 2, 3, and 4, where a higher grade score indicates increased spasticity [33]. According to the MAS scoring system, the selected children were graded 1+and 2 which are the most available grades in the outpatient clinic during this study.
Statistical analysis
The relationship between BMI, SVMC, and the level of functional independence in children with diplegic CP was assessed using correlation analysis. The statistical package for the social sciences program, version 25 (IBM SPSS, Chicago, IL, USA) was used for analyses. Descriptive statistics were expressed in numerical (n) and percent (%) formats, and mean±standard deviation. Spearman’s correlation coefficient was selected to detect the relationships due to the ordinal nature of the data. A correlation was considered significant at a p<0.05.
Results
In this study, 84 children with spastic diplegic CP were selected. Table 1 displays the general characteristics of the children participating in the study. There was a positive, strong, and significant correlation between MAS and BMI (rs=+0.677, P<0.001); between WEE FIM total score and SCALE (rs=+0.718, P<0.001); between the physical dimension of WEE FIM and SCALE (rs=+0.843, P<0.001); and between the cognitive dimension of WEE FIM and SCALE (rs=+0.904, P<0.001), while there was a negative, strong, and significant correlation between MAS and WEE FIM total score (rs=-0.669, P<0.001), between MAS and the physical dimension of WEE FIM (rs=-0.712, P<0.001), between MAS and the cognitive dimension of WEE FIM, (rs=-0.793, P<0.001), between MAS and SCALE (rs=-0.830, P<0.001), between the cognitive dimension of WEE FIM and GMFCS(rs=-0.660, P<0.001), between SCALE and GMFCS (rs=-0.707, P<0.001), and between WEE FIM total score and BMI (rs=-0.607, P<0.001) as presented in Table 2.

In addition, there was a positive, moderate, and significant correlation between MAS and GMFCS (rs=+0.539, P<0.001) and between BMI and GMFCS (rs=+0.53, P<0.032). There was a negative, moderate, and significant correlation between GMFCS and WEE FIM total score(rs=-0.540, P<0.001), between GMFCS and the physical dimension of WEE FIM (rs=-0.531, P<0.001), between BMI and SCALE(rs=-0.484, P<0.001), between BMI and the physical dimension of WEEFIM (rs=-0.537, P<0.001), and between BMI and the cognitive dimension of WEE FIM (rs=-0.460, P<0.001), as presented in Table 2.
Regarding the WEE FIM scale, there was a positive, strong, and significant correlation between the WEE FIM total score with its physical and cognitive dimensions (rs=+0.803, P<0.001), (rs=+0.752, P=0.001) respectively, and between the physical and cognitive dimensions of WEE FIM scale (rs=+0.837, P<0.001), as presented in Table 2.
Discussion
Children with spastic diplegia have disabilities of the lower extremities resulting in dependency on their functional daily activities. Spasticity, muscle weakness, and activity limitations contribute to a lack of isolated motor control, limitations in their daily living activities, and obesity in some children [34, 35]. The findings of this study showed a significant correlation between BMI, SVMC, functional independence, gross motor function, and spasticity in the participating children.
Many studies suggest different mechanisms by which children with CP are liable to become obese. One such mechanism is that children with CP often are premature babies which is associated with obesity [36]. In addition, children with CP face unique difficulties in the issues related to physical activity and functional ability. So, it is not surprising that children with CP have a higher prevalence of overweight and obesity compared to their normal peers, especially the ones who can walk [21, 37]. In parallel with these studies, this study showed a significant negative correlation between functional independence and BMI.
The body composition of CP is affected by dietary components and also non-nutritional aspects such as impaired mobility, atypical movement patterns, motor impairment severity, and type of CP, that affect obesity. Karatekin et al. [38] mentioned that normal children had a lower obesity rate when compared to ambulant children with CP. In addition, they found that gross motor function was significantly associated with BMI and weight.
Oftedal et al. [16] added that children with GMFCS levels II–V have a higher body fat percentage than those children with GMFCS level I, which is consistent with the positive significant correlation between GMFCS E&R and BMI in this study. So, it is important to measure and follow BMI during the evaluation and rehabilitation of children with diplegic CP when the goal is to improve their functional independence and gross motor functions.
A higher BMI is associated with diminished executive functions and a thinner prefrontal cortex in children [39]. Meo et al. [40] observed significant impairments in cognitive functions in markedly obese students. This comes in agreement with the results of this study which reported a negative significant correlation between BMI and the cognitive dimension of WEE FIM.
The response to physical activity in children with CP may vary by the motor impairment degree and the type of the disorder. So, in contrast to the results of this study which showed a positive significant correlation between GMFCS E&R and BMI, Arshad and Arora [41] showed a negative correlation. This can be illustrated by including all five levels of GMFCS E&R in their study, not only levels II and III.
Being active is more difficult for children with CP who have more severe motor affection than children who have mild impairment. They may have cognitive, perceptive, behavioral, or communication disorders in addition to disorders of muscle tone and strength, that negatively affect their functional ability in different ways [42]. This supports the results of the current study which found a negative significant correlation between gross motor function level with both functional ability and SVMC. Arruda et al. [42] added that in addition to the reduction in muscle strength and high muscle tone, other factors can likely explain more affected functional ability among children with greater motor impairment such as skeletal muscle deformities and more frequent use of medications. This can illustrate the negative significant correlation between functional independence with both spasticity and gross motor function level in this study.
Reduced selective motor control and increased resistance to joint movement are important factors that influence gross motor function. Noble et al. [8] found a positive significant correlation between gross motor function and selectivity (r=0.901), and a negative significant correlation between gross motor function and MAS (r=-0.691), which are in agreement with the results of this study.
Children with CP often have low or normal muscle tone at the beginning of their life which increases in most children up to 5 years of age [43]. For this reason, children’s age in this study was selected to start from 6 years.
Selective motor control disorders are affected by brain damage and are difficult to improve. Thus, SVMC is an important factor that affects motor function in children with CP. In this study, there was a negative strong correlation between SCALE scores and levels of gross motor function which is similar to findings of previous studies of SVMC in children with CP which reported that SCALE is strongly and significantly correlated with GMFM scale in children with bilateral CP [44, 45].
Conclusion
This study shows a significant relationship between BMI, SVMC, and functional independence in children with spastic diplegia. These results could have a remarkable future effect in educational, experimental, and clinical settings. This study provides evidence that BMI and SVMC impairments should be included while studying and clinically evaluating children with diplegic CP. Therapeutic intervention of SVMC should be carefully considered when planning a program to increase the functional independence of children with spastic diplegia. Also, regular height and weight measurement is an important factor in the rehabilitation programs that aim to increase functional abilities in these children.
Limitations
The study included only children with diplegic CP, which may restrict the findings’ generalizability to other types of CP. The study included only children with GMFCS E&R levels II and III, excluding severely disabled children which may limit the findings’ generalizability to other levels of GMFCS E&R. The study used MAS to assess spasticity, which has been questioned for its validity. More advanced measurements of spasticity, such as electromyography, may provide more accurate assessments of spasticity. Finally, the study did not account for the effects of medication use on the study outcomes, which may have confounded the results.
Ethical Considerations
Compliance with ethical guidelines
Children were only allowed to be included in this study after having a signed written consent form from their parents. The clinical trial registration number of this study is NCT05428865. The research has followed the tenets of the Declaration of Helsinki and has been approved by the Faculty of Physical Therapy; Cairo University (No.: P.T.REC/012/003833).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
Acknowledgments
The author would like to thank all the children who participated in the study and their parents.
References
- Patel DR, Neelakantan M, Pandher K, Merrick J. Cerebral palsy in children: A clinical overview. Translational Pediatrics. 2020; 9(Suppl 1):S125-35. [DOI:10.21037/TP.2020.01.01.] [PMID]
- McIntyre S, Goldsmith S, Webb A, Ehlinger V, Hollung SJ, McConnell K, et al. Global prevalence of cerebral palsy: A systematic analysis. Developmental Medicine and Child Neurology. 2022; 64(12):1494-506. [PMID]
- Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Developmental Medicine and Child Neurology. 2013; 55(6):509-19. [DOI:10.1111/DMCN.12080] [PMID]
- Bourgleh S, Nemeş RN, Hetaimish B, Burileanu AH, Fallatah S, Chiuţu LC. Cerebral palsy. Considerations upon 249 consecutive patients and review of literature. Current Health Sciences Journal. 2019; 45(4):405. [DOI:10.12865/CHSJ.45.04.09]
- Zhou JY, Lowe E, Cahill-Rowley K, Mahtani GB, Young JL, Rose J. Influence of impaired selective motor control on gait in children with cerebral palsy. Journal of Children's Orthopaedics. 2019; 13(1):73-81. [DOI:10.1302/1863-2548.13.180013] [PMID]
- Sanger TD, Chen D, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW, et al. Definition and classification of negative motor signs in childhood. Pediatrics. 2006; 118(5):2159-67. [DOI:10.1542/PEDS.2005-3016] [PMID]
- Fahr A, Keller JW, van Hedel HJA. A systematic review of training methods that may improve selective voluntary motor control in children with spastic cerebral palsy. Frontiers in Neurology. 2020; 11:572038. [PMID]
- Noble JJ, Gough M, Shortland AP. Selective motor control and gross motor function in bilateral spastic cerebral palsy. Developmental Medicine and Child Neurology. 2018; 61(1):57-61. [DOI:10.1111/dmcn.14024] [PMID]
- Cahill-Rowley K, Rose J. Etiology of impaired selective motor control: Emerging evidence and its implications for research and treatment in cerebral palsy. Developmental Medicine and Child Neurology. 2014; 56(6):522-8. [DOI:10.1111/dmcn.12355] [PMID]
- Kiernan D, Nikolopoulou C, Brady K. Prevalence of overweight and obesity in Irish ambulant children with cerebral palsy. Irish Journal of Medical Science. 2021; 190(1):225-31. [DOI:10.1007/S11845-020-02294-4] [PMID]
- Rogozinski BM, Davids JR, Davis RB, Christopher LM, Anderson JP, Jameson GG, et al. Prevalence of obesity in ambulatory children with cerebral palsy. The Journal of Bone and Joint Surgery. American Volume. 2007; 89(11):2421-6. [DOI:10.2106/JBJS.F.01080] [PMID]
- Bansal A, Diwan S, Diwan J, Vyas N. Prevalance of obesity in children with cerebral palsy. Journal of Clinical and Diagnostic Research: JCDR. 2014; 8(8):BC08-11. [DOI:10.7860/JCDR/2014/8462.4679] [PMID]
- Duran I, Schulze J, Martakis KS, Stark C, Schoenau E. Diagnostic performance of body mass index to identify excess body fat in children with cerebral palsy. Developmental Medicine and Child Neurology. 2018; 60(7):680-6. [DOI:10.1111/DMCN.13714/ABSTRACT] [PMID]
- World Health Organization (WHO). Body mass index (BMI) [Internet]. 2023 [Updated 2023 April 14]. Available from: [Link]
- Hurvitz EA, Green LB, Hornyak JE, Khurana SR, Koch LG. Body mass index measures in children with cerebral palsy related to gross motor function classification: A clinic-based study. American Journal of Physical Medicine & Rehabilitation. 2008; 87(5):395-403. [DOI:10.1097/PHM.0B013E3181617736] [PMID]
- Oftedal S, Davies PS, Boyd RN, Stevenson RD, Ware RS, Keawutan P, et al. Body composition, diet, and physical activity: A longitudinal cohort study in preschoolers with cerebral palsy. The American Journal of Clinical Nutrition. 2017; 105(2):369-78. [DOI:10.3945/AJCN.116.137810] [PMID]
- D'Souza A, Bolsterlee B, Lancaster A, Herbert RD. Intramuscular fat in children with unilateral cerebral palsy. Clinical Biomechanics (Bristol, Avon). 2020; 80:105183. [DOI:10.1016/J.CLINBIOMECH.2020.105183] [PMID]
- Walker JL, Bell KL, Stevenson RD, Weir KA, Boyd RN, Davies PS. Differences in body composition according to functional ability in preschool-aged children with cerebral palsy. Clinical Nutrition. 2015; 34(1):140–5. [PMID]
- Papageorgiou E, Simon-Martinez C, Molenaers G, Ortibus E, Van Campenhout A, Desloovere K. Are spasticity, weakness, selectivity, and passive range of motion related to gait deviations in children with spastic cerebral palsy? A statistical parametric mapping study. PLoS One. 2019; 14(10):e0223363. [DOI:10.1371/JOURNAL.PONE.0223363] [PMID]
- Chruscikowski E, Fry NRD, Noble JJ, Gough M, Shortland AP. Selective motor control correlates with gait abnormality in children with cerebral palsy. Gait & Posture. 2017; 52:107-9. [DOI:10.1016/j.gaitpost.2016.11.031] [PMID]
- Şimşek TT, Tuç G. Examination of the relation between body mass index, functional level and health-related quality of life in children with cerebral palsy. Turk Pediatri Arsivi. 2014; 49(2):130-7. [DOI:10.5152/TPA.2014.1238] [PMID]
- Kuczmarski Robert J. CDC growth charts: United States. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2000. [Link]
- Sukal-Moulton T, Fowler E. Selective voluntary motor control in children and youth with spastic cerebral palsy. In: Miller F, Bachrach S, Lennon N, O'Nei ME, editors. Cerebral palsy. Cham: Springer Nature; 2020. [DOI:10.1007/978-3-319-74558-9_162]
- Kim GW, Kim H, Jeon JY, Jang JS. Validity and reliability of functional independence measure for children (WeeFIM) for children with cerebral palsy. Inquiry: A Journal of Medical Care Organization, Provision and Financing. 2022; 59:469580211072454. [DOI:10.1177/00469580211072454] [PMID]
- Mohamed Othman E, Aly DA. To what extent the Arabic WeeFIM is reliable and feasible in Egyptian children With burns? An observational cross-sectional study. Journal of Burn Care & Research: Official Publication of the American Burn Association. 2023; 44(3):590-8. [DOI:10.1093/JBCR/IRAC094] [PMID]
- Park EY, Kim WH, Choi YI. Factor analysis of the WeeFIM in children with spastic cerebral palsy. Disability and Rehabilitation. 2013; 35(17):1466-71. [PMID]
- Bodkin AW, Robinson C, Perales FP. Reliability and validity of the gross motor function classification system for cerebral palsy. Pediatric Physical Therapy: The Official Publication of the Section on Pediatrics of the American Physical Therapy Association. 2003; 15(4):247-52. [DOI:10.1097/01.PEP.0000096384.19136.02] [PMID]
- Almasri N, Saleh M. Inter-rater agreement of the Arabic Gross Motor Classification System Expanded & Revised in children with cerebral palsy in Jordan. Disability and Rehabilitation. 2015; 37(20):1895-901. [DOI:10.3109/09638288.2014.986589] [PMID]
- Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Developmental Medicine and Child Neurology. 2008; 50(10):744-50. [DOI:10.1111/J.1469-8749.2008.03089.X] [PMID]
- Jansheski G. Cerebral Palsy Gross Motor Classification System [Internet]. 2023 [Updated 2022 April 23]. Available from: [Link]
- Karen Pape, MD. Diplegia topography GMFCS I to III [Internet]. 2023 [Updated 2023 April 13]. Available from: [Link]
- Pascoe J, Thomason P, Graham HK, Reddihough D, Sabin MA. Body mass index in ambulatory children with cerebral palsy: A cohort study. Journal of Paediatrics and Child Health. 2016; 52(4):417-21. [DOI:10.1111/JPC.13097] [PMID]
- Harb A, Kishner S. Modified Ashworth scale. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. [PMID]
- Pouliot-Laforte A, Parent A, Hamdy R, Marois P, Lemay M, Ballaz L. Relationship between lower limb strength and walking capacities in children with spastic bilateral cerebral palsy. Disability and Rehabilitation. 2022; 44(10):1916-22. [PMID]
- Kusumoto Y, Takaki K, Matsuda T, Nitta O. Relation of selective voluntary motor control of the lower extremity and extensor strength of the knee joint in children with spastic diplegia. Journal of Physical Therapy Science. 2016; 28(6):1868-71. [DOI:10.1589/JPTS.28.1868] [PMID]
- Gurka MJ, Kuperminc MN, Busby MG, Bennis JA, Grossberg RI, Houlihan CM, et al. Assessment and correction of skinfold thickness equations in estimating body fat in children with cerebral palsy. Developmental Medicine and Child Neurology. 2010; 52(2):e35-41. [DOI:10.1111/j.1469-8749.2009.03474.x] [PMID]
- Stevenson RD. Body mass index and obesity in children with cerebral palsy. Developmental Medicine & Child Neurology. 2018; 60(7):639. [PMID]
- Karatekin BD, Kacar G, Icagasioglu A. Obesity in ambulatory children with cerebral palsy in Turkey: A cross-sectional study. Journal of Pediatric Rehabilitation Medicine. 2023; 16(1):195-202. [DOI:10.3233/PRM-210093] [PMID]
- Laurent JS, Watts R, Adise S, Allgaier N, Chaarani B, Garavan H, et al. Associations among body mass index, cortical thickness, and executive function in children. JAMA Pediatrics. 2020; 174:170-7. [DOI:10.1001/JAMAPEDIATRICS.2019.4708] [PMID]
- Meo SA, Altuwaym AA, Alfallaj RM, Alduraibi KA, Alhamoudi AM, Alghamdi SM, et al. Effect of obesity on cognitive function among school adolescents: A cross-sectional study. Obesity Facts. 2019; 12(2):1500-6. [DOI:10.1159/000499386] [PMID]
- Arshad S, Arora S. Co-Relation between body mass index and gross motor function classification in children with cerebral palsy. Journal of Medical and Dental Science Research. 2021; 8(8):55-8. [Link]
- Arruda RCBF, Tassitano RM, da Silva Brito AL, de Sousa Martins OS, Cabral PC, de Castro Antunes MM. Physical activity, sedentary time and nutritional status in Brazilian children with cerebral palsy. Jornal de pediatria. 2022; 98(3):303-9. [DOI:10.1016/J.JPED.2021.07.005] [PMID]
- Lindén O, Hägglund G, Rodby-Bousquet E, Wagner P. The development of spasticity with age in 4,162 children with cerebral palsy: A register-based prospective cohort study. Acta orthopaedica. 2019; 90(3):286-91. [DOI:10.1080/17453674.2019.1590769] [PMID]
- Østensjø S, Carlberg EB, Vøllestad NK. Motor impairments in young children with cerebral palsy: Relationship to gross motor function and everyday activities. Developmental Medicine and Child Neurology. 2004; 46(9):580-9. [PMID]
- Yun G, Huang M, Cao J, Hu X. Selective motor control correlates with gross motor ability, functional balance and gait performance in ambulant children with bilateral spastic cerebral palsy. Gait & Posture. 2023; 99:9-13. [DOI:10.1016/J.GAITPOST.2022.10.009] [PMID]
Article type: Original Research Articles |
Subject:
Physiotherapy
Received: 2022/11/10 | Accepted: 2023/09/5 | Published: 2023/12/1
Received: 2022/11/10 | Accepted: 2023/09/5 | Published: 2023/12/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








