Volume 16, Issue 3 (September 2018)
Iranian Rehabilitation Journal 2018, 16(3): 265-270 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aliasgharpour M, Arazi T, Mohammadi S, Mohammadi N, Kazemnejad A. Effect of Incentive Spirometry on Pulmonary Function Tests in Patients Undergoing Hemodialysis: A Randomized Clinical Trials. Iranian Rehabilitation Journal 2018; 16 (3) :265-270
URL: http://irj.uswr.ac.ir/article-1-813-en.html
URL: http://irj.uswr.ac.ir/article-1-813-en.html
Mansooreh Aliasgharpour1 

 , Tajmohammad Arazi *2
, Tajmohammad Arazi *2 

 , Sepideh Mohammadi3
, Sepideh Mohammadi3 

 , Nooredin Mohammadi4
, Nooredin Mohammadi4 

 , Anoushirvan Kazemnejad5
, Anoushirvan Kazemnejad5 




 , Tajmohammad Arazi *2
, Tajmohammad Arazi *2 

 , Sepideh Mohammadi3
, Sepideh Mohammadi3 

 , Nooredin Mohammadi4
, Nooredin Mohammadi4 

 , Anoushirvan Kazemnejad5
, Anoushirvan Kazemnejad5 


1- Department of Medical Surgical Nursing, Faculty of Nursing and Midwifery, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Operating Room, Neyshabur University of Medical Sciences, Neyshabur, Iran.
3- Department of Nursing, Nursing Care Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
4- Department of Nursing, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Biostatistics, School of Medicine, Tarbiat Modares University, Tehran, Iran.
2- Department of Operating Room, Neyshabur University of Medical Sciences, Neyshabur, Iran.
3- Department of Nursing, Nursing Care Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
4- Department of Nursing, School of Nursing and Midwifery, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Biostatistics, School of Medicine, Tarbiat Modares University, Tehran, Iran.
Full-Text [PDF 545 kb]
(3139 Downloads)
| Abstract (HTML) (5816 Views)
Full-Text: (2149 Views)
1. Introduction
Chronic Renal Failure (CRF) is a complicated disease and is rapidly increasing worldwide. It is among major causes of death and disability [1]. CRF is a systemic disorder with destructive effects on all organs [2]. The kidneys perform many crucial functions including regulation of water and blood pressure, and acid-base and electrolyte balance, and participation in hormones functions. Patients with CRF require hemodialysis or peritoneal dialysis for survival, because these can partially replace the impaired kidney function [3]. Most patients with CRF are treated with hemodialysis [4]. While hemodialysis can replace some of the lost function of kidneys [5], some patients develop multiple complications in the musculoskeletal, cardiovascular, metabolic and respiratory systems [3].
Pulmonary complication is common in CRF patients and those on hemodialysis [6, 7]. Pulmonary edema is common in chronic dialysis patients. Factors such as permeability of the capillaries, intravascular and interstitial volume overload, high blood pressure, and congestive heart failure increase the risk of occurrence and progression of pulmonary edema in CRF. These changes alter physiological and mechanical function of the lungs and subsequent increase in airway resistance [8]. Also most patients on hemodialysis experience some degree of hypoxemic hypoxia at the beginning or towards the end of treatment session. Respiratory failure secondary to hyperkalemia, pleural effusion, and pulmonary hypertension is life threatening in chronic hemodialysis [9].
Mallamaci et al. indicated that pulmonary congestion is highly prevalent in symptomatic and asymptomatic hemodialysis patients [10]. Also Mousavi et al. demonstrated a surprisingly high prevalence of pulmonary hypertension among patients with end-stage renal disease receiving hemodialysis, in Iran [11]. In fact, some authors have reported that 75% of patients on long-term hemodialysis present restrictive spirometry abnormalities [12]. Cury et al. indicated that patients with CRF on dialysis showed impaired functional capacity such as FVC and FEV1 and lung function impairment [3]. Although the incidence of pulmonary complications in chronic hemodialysis patients is high, studies in this area are overlooked in Iran.
Incentive spirometry is a safe way for treatment of patient with pulmonary dysfunction. The effect of incentive spirometry on pulmonary ventilation and function is investigated in different diseases [13-17]. There is no consensus about the effect of incentive spirometry on pulmonary function tests [13]. Therefore, this study aimed to evaluate the effect of incentive spirometry on pulmonary function tests in patients on hemodialysis.
2. Methods
Study setting and participants
This was a randomized clinical trial conducted on hemodialysis patients in Hasheminejhad Hospital, in Tehran, Iran. The inclusion criteria comprised having Forced Vital Capacity (FVC) and forced expiratory volume during the first second of forced expiration (FEV1) of at least 15% less than the normal ranges (according to the age and height of the patient) [18], being able to perform spirometry, having normal chest and vertebral column, being 18 years of age or older, being Persian literate, receiving hemodialysis 3 times a week for at least 6 months. The exclusion criteria were having the history of respiratory diseases such as asthma and tuberculosis or current respiratory infections. Samples were selected by a random number Table, then divided into the experimental and control groups by a Randomized Block Design. Age, gender, height and duration of hemodialysis were considered as the blocking factors.
According to Haeffener et al. [19] with 95% confidence interval and 99% power, 16.4~17 patients were enrolled in each group due to the difference of 4% FVC and FEV1 in the intervention and control groups and based on the following formula. A total of 52 patients (26 per group) participated in the study, considering a 50% sample loss.
Chronic Renal Failure (CRF) is a complicated disease and is rapidly increasing worldwide. It is among major causes of death and disability [1]. CRF is a systemic disorder with destructive effects on all organs [2]. The kidneys perform many crucial functions including regulation of water and blood pressure, and acid-base and electrolyte balance, and participation in hormones functions. Patients with CRF require hemodialysis or peritoneal dialysis for survival, because these can partially replace the impaired kidney function [3]. Most patients with CRF are treated with hemodialysis [4]. While hemodialysis can replace some of the lost function of kidneys [5], some patients develop multiple complications in the musculoskeletal, cardiovascular, metabolic and respiratory systems [3].
Pulmonary complication is common in CRF patients and those on hemodialysis [6, 7]. Pulmonary edema is common in chronic dialysis patients. Factors such as permeability of the capillaries, intravascular and interstitial volume overload, high blood pressure, and congestive heart failure increase the risk of occurrence and progression of pulmonary edema in CRF. These changes alter physiological and mechanical function of the lungs and subsequent increase in airway resistance [8]. Also most patients on hemodialysis experience some degree of hypoxemic hypoxia at the beginning or towards the end of treatment session. Respiratory failure secondary to hyperkalemia, pleural effusion, and pulmonary hypertension is life threatening in chronic hemodialysis [9].
Mallamaci et al. indicated that pulmonary congestion is highly prevalent in symptomatic and asymptomatic hemodialysis patients [10]. Also Mousavi et al. demonstrated a surprisingly high prevalence of pulmonary hypertension among patients with end-stage renal disease receiving hemodialysis, in Iran [11]. In fact, some authors have reported that 75% of patients on long-term hemodialysis present restrictive spirometry abnormalities [12]. Cury et al. indicated that patients with CRF on dialysis showed impaired functional capacity such as FVC and FEV1 and lung function impairment [3]. Although the incidence of pulmonary complications in chronic hemodialysis patients is high, studies in this area are overlooked in Iran.
Incentive spirometry is a safe way for treatment of patient with pulmonary dysfunction. The effect of incentive spirometry on pulmonary ventilation and function is investigated in different diseases [13-17]. There is no consensus about the effect of incentive spirometry on pulmonary function tests [13]. Therefore, this study aimed to evaluate the effect of incentive spirometry on pulmonary function tests in patients on hemodialysis.
2. Methods
Study setting and participants
This was a randomized clinical trial conducted on hemodialysis patients in Hasheminejhad Hospital, in Tehran, Iran. The inclusion criteria comprised having Forced Vital Capacity (FVC) and forced expiratory volume during the first second of forced expiration (FEV1) of at least 15% less than the normal ranges (according to the age and height of the patient) [18], being able to perform spirometry, having normal chest and vertebral column, being 18 years of age or older, being Persian literate, receiving hemodialysis 3 times a week for at least 6 months. The exclusion criteria were having the history of respiratory diseases such as asthma and tuberculosis or current respiratory infections. Samples were selected by a random number Table, then divided into the experimental and control groups by a Randomized Block Design. Age, gender, height and duration of hemodialysis were considered as the blocking factors.
According to Haeffener et al. [19] with 95% confidence interval and 99% power, 16.4~17 patients were enrolled in each group due to the difference of 4% FVC and FEV1 in the intervention and control groups and based on the following formula. A total of 52 patients (26 per group) participated in the study, considering a 50% sample loss.
Intervention
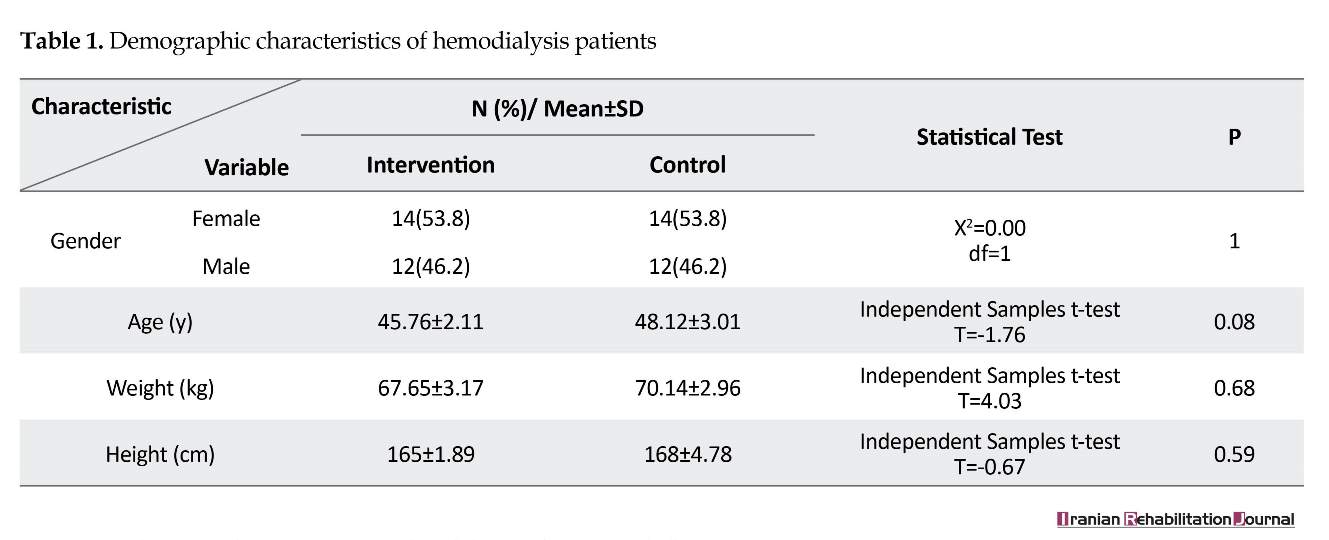
Fifty-two hemodialysis patients were assigned to the experimental (n=26) and control (n=26) groups (Table 1). All study participant were hemodialysis patients referred to the hospital three times a week for hemodialysis. The control group, received the routine treatment provided by the hospital such as general medical and nursing care and their dialytic care (checking the patient's weight and nutrition). We measured the pulmonary function of all patients in both groups at the onset of the study. Pulmonary care and educating patients about the prevention of pulmonary dysfunction was not routine.
The experimental group received routine hospital services in addition to the incentive spirometry program. The experimental group were initially trained for incentive spirometry by the researcher. After a quiet expiration, the patient was encouraged to take gradual and slow maximal inspirations through the mouthpiece of the device and hold each breath for as long as possible. The patients were instructed to use the device 5–10 breaths per session at a minimum, every hour while awake.
The administration of incentive spirometry started at the beginning of study for every patient and continued for 2 months at home [14]. Also, we provided them an incentive spirometer with a pictorial educational booklet containing all the verbally-provided training materials. All incentive spirometers provided to the intervention group were Shree Chem or similar brand. The routine pulmonary function test including FVC, FEV1, FEV1/FVC were demonstrated at the beginning and 2 month after the initiation of the study to both groups. All patients were evaluated by the pulmonary function test before hemodialysis treatment in the hospital. The pulmonary function was evaluated by a spirometer (Model: Flowhandy ZAN type: 3.1) in both groups. We used the same spirometer during the study and its reliability was assessed by the test-retest method. For this reason, FVC, FEV1, FEV1/FVC were evaluated in 15 patients for 2 times within 5 minutes under the same conditions, and the correlation coefficient was 95%.
Data analysis
The obtained data were analyzed by SPSS version 18. P<0.05 was considered as statistically significant. Descriptive statistics such as mean, standard deviation, percentage, etc. were used to analyze the samples characteristics. The Independent t-test and the Chi-square test were used to identify the differences among the groups.
3. Results
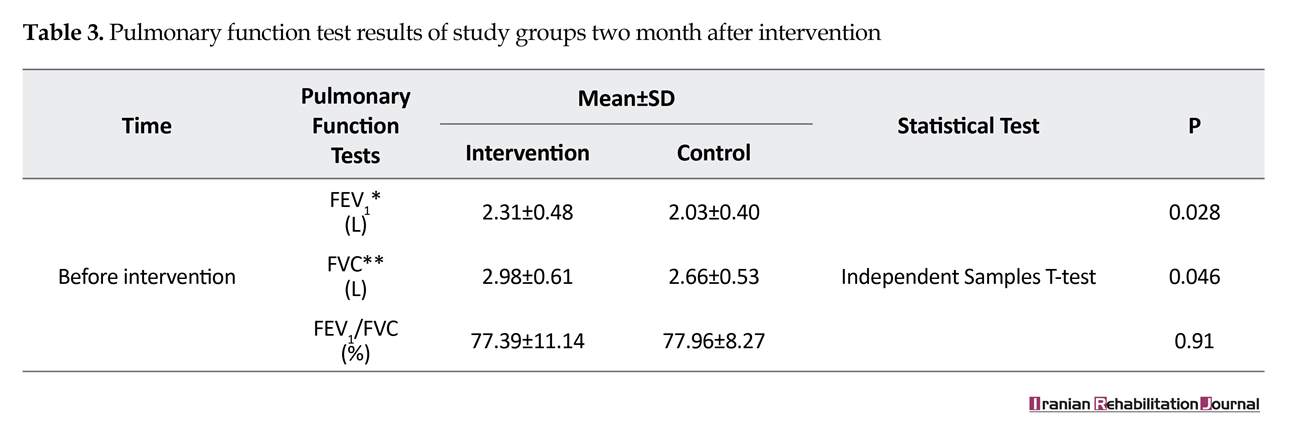
Most of the patients were female (53.82%), aged between 40 and 49 years (48.07%), weighted between 60 and 79 kg (53.84%) and with the height between 161 and 175 cm (55.76%). There was no significant differences between the two groups in terms of demographic characteristics (Table 1). There were no significant differences in FVC, FEV1, FEV1/FVC between the patients in the experimental and control groups before the intervention (Table 2). The FVC and FEV1 increased significantly in the intervention group compared to the control group (P<0.5) after two months, while no significant improvement was observed in the FEV1/FVC ratio (Table 3).
4. Discussion
Our study showed that the incentive spirometry had a positive effect on pulmonary function tests (FVC, FEV1) in patients on hemodialysis. An incentive spirometer is a
Fifty-two hemodialysis patients were assigned to the experimental (n=26) and control (n=26) groups (Table 1). All study participant were hemodialysis patients referred to the hospital three times a week for hemodialysis. The control group, received the routine treatment provided by the hospital such as general medical and nursing care and their dialytic care (checking the patient's weight and nutrition). We measured the pulmonary function of all patients in both groups at the onset of the study. Pulmonary care and educating patients about the prevention of pulmonary dysfunction was not routine.
The experimental group received routine hospital services in addition to the incentive spirometry program. The experimental group were initially trained for incentive spirometry by the researcher. After a quiet expiration, the patient was encouraged to take gradual and slow maximal inspirations through the mouthpiece of the device and hold each breath for as long as possible. The patients were instructed to use the device 5–10 breaths per session at a minimum, every hour while awake.
The administration of incentive spirometry started at the beginning of study for every patient and continued for 2 months at home [14]. Also, we provided them an incentive spirometer with a pictorial educational booklet containing all the verbally-provided training materials. All incentive spirometers provided to the intervention group were Shree Chem or similar brand. The routine pulmonary function test including FVC, FEV1, FEV1/FVC were demonstrated at the beginning and 2 month after the initiation of the study to both groups. All patients were evaluated by the pulmonary function test before hemodialysis treatment in the hospital. The pulmonary function was evaluated by a spirometer (Model: Flowhandy ZAN type: 3.1) in both groups. We used the same spirometer during the study and its reliability was assessed by the test-retest method. For this reason, FVC, FEV1, FEV1/FVC were evaluated in 15 patients for 2 times within 5 minutes under the same conditions, and the correlation coefficient was 95%.
Data analysis
The obtained data were analyzed by SPSS version 18. P<0.05 was considered as statistically significant. Descriptive statistics such as mean, standard deviation, percentage, etc. were used to analyze the samples characteristics. The Independent t-test and the Chi-square test were used to identify the differences among the groups.
3. Results
Most of the patients were female (53.82%), aged between 40 and 49 years (48.07%), weighted between 60 and 79 kg (53.84%) and with the height between 161 and 175 cm (55.76%). There was no significant differences between the two groups in terms of demographic characteristics (Table 1). There were no significant differences in FVC, FEV1, FEV1/FVC between the patients in the experimental and control groups before the intervention (Table 2). The FVC and FEV1 increased significantly in the intervention group compared to the control group (P<0.5) after two months, while no significant improvement was observed in the FEV1/FVC ratio (Table 3).
4. Discussion
Our study showed that the incentive spirometry had a positive effect on pulmonary function tests (FVC, FEV1) in patients on hemodialysis. An incentive spirometer is a
medical device designed to achieve and maintain maximal inspiration and help keeping the lungs as healthy as possible. An incentive spirometer is used to help patients improve their respiration function. It is an easy-to-use device for patients. Visual feedback on airflow and volume is among important advantages of this device. Its prolonged use results in effective inspiration, more controlled flow, and greater enthusiasm to practice [17].
We found that two-month use of incentive spirometer improved pulmonary function (FVC, FEV1) in hemodialysis patients, and incentive spirometer could be an effective device for improving lung function in hemodialysis patients. However, the efficacy of incentive spirometry remains controversial [13, 15]. Kotani et al. indicated that using incentive spirometer for two weeks improved respiratory motion and lung function in healthy individuals, and they suggested that incentive spirometer may be an effective preoperative medical device for modifying pulmonary function during the perioperative period [20].
Thomas and McIntosh in a systematic review and meta-analysis found that deep breathing practice by incentive spirometer appeared to be more useful than no physical therapy intervention in preventing postoperative pulmonary problems [16]. However, Agostini et al. indicated that incentive spirometry did not affect the frequency of
We found that two-month use of incentive spirometer improved pulmonary function (FVC, FEV1) in hemodialysis patients, and incentive spirometer could be an effective device for improving lung function in hemodialysis patients. However, the efficacy of incentive spirometry remains controversial [13, 15]. Kotani et al. indicated that using incentive spirometer for two weeks improved respiratory motion and lung function in healthy individuals, and they suggested that incentive spirometer may be an effective preoperative medical device for modifying pulmonary function during the perioperative period [20].
Thomas and McIntosh in a systematic review and meta-analysis found that deep breathing practice by incentive spirometer appeared to be more useful than no physical therapy intervention in preventing postoperative pulmonary problems [16]. However, Agostini et al. indicated that incentive spirometry did not affect the frequency of
postoperative pulmonary complications and overall did not progress the recovery of respiratory function or influence the length of stay [21]. In a systematic review, Carvalho et al. [15] concluded that there was not sufficient evidence to support using incentive spirometer in the management of surgical patients in order to provide visual feedback of airflow and volume. The usage of it results in a long period of effective inspiration, higher enthusiasm to practice, and more controlled flow [17].
Most studies in this area concluded that one reason for the effectiveness of incentive spirometry in pulmonary function is continuous and correct implementation of incentive spirometer. The experimental group of our study were encouraged to use the device for 5–10 breaths per session, at a minimum, every hour while awake for two months. Maybe consistency in the implementation of incentive spirometer and 2 months duration of its use was the strength and advantage to the effectiveness of our study. Navari et al. indicated that dialysis with bicarbonate dialysate versus acetate dialysates causes significant improvements in spirometry parameters in men on maintenance dialysis [22]. Thus, maybe some factors (e.g. type of dialysates) influenced the interpretation of our findings and this could be a limitation of the study.
5. Conclusion
This study showed significant differences in pulmonary function between the two groups. Therefore, incentive spirometer seems to be a safe medical tool and an effective choice for improving the pulmonary function test in hemodialysis patients. However, considering the limited studies available, further research is required in this area.
Ethical Considerations
Compliance with ethical guidelines
The project was approved by the Ethics Committee of Tehran University of Medical Sciences and the informed consent form was obtained from all of the study participants. All participants were informed that their participation was voluntary and the study would not induce any physical harm. We ensured strict anonymity and confidentiality. Our study was confirmed under the Iranian Registry of Clinical Trials (IRCT2012080522226N10).
Funding
This research has been supported by Tehran University of Medical Sciences & a health services grant.
Conflicts of interest
The authors certify that they have no affiliation with or involvement in any organization or entity with any financial, or non- financial interest in the subject matter or materials dismissed in this manuscript.
Acknowledgments
The authors would like to thank the nurses and physicians for their participation in this study. Special thanks go to the patients who devoted their time to make this project a success.
References
Most studies in this area concluded that one reason for the effectiveness of incentive spirometry in pulmonary function is continuous and correct implementation of incentive spirometer. The experimental group of our study were encouraged to use the device for 5–10 breaths per session, at a minimum, every hour while awake for two months. Maybe consistency in the implementation of incentive spirometer and 2 months duration of its use was the strength and advantage to the effectiveness of our study. Navari et al. indicated that dialysis with bicarbonate dialysate versus acetate dialysates causes significant improvements in spirometry parameters in men on maintenance dialysis [22]. Thus, maybe some factors (e.g. type of dialysates) influenced the interpretation of our findings and this could be a limitation of the study.
5. Conclusion
This study showed significant differences in pulmonary function between the two groups. Therefore, incentive spirometer seems to be a safe medical tool and an effective choice for improving the pulmonary function test in hemodialysis patients. However, considering the limited studies available, further research is required in this area.
Ethical Considerations
Compliance with ethical guidelines
The project was approved by the Ethics Committee of Tehran University of Medical Sciences and the informed consent form was obtained from all of the study participants. All participants were informed that their participation was voluntary and the study would not induce any physical harm. We ensured strict anonymity and confidentiality. Our study was confirmed under the Iranian Registry of Clinical Trials (IRCT2012080522226N10).
Funding
This research has been supported by Tehran University of Medical Sciences & a health services grant.
Conflicts of interest
The authors certify that they have no affiliation with or involvement in any organization or entity with any financial, or non- financial interest in the subject matter or materials dismissed in this manuscript.
Acknowledgments
The authors would like to thank the nurses and physicians for their participation in this study. Special thanks go to the patients who devoted their time to make this project a success.
References
- Tamadon MR, Adibimehr AR, Ghorbani R. Cognitive impairment in dialysis and non-dialysis patients suffering from chronic renal failure and comparing them with a control group. Middle East Journal of Rehabilitation and Health Studies. 2017; 4(4):e12815. [DOI:10.5812/mejrh.12815]
- Durgun B, Yüksel A, Erol G, Kobuk M, Doğancı S. Acute on chronic renal failure has worse postoperative outcomes than end-stage renal disease following cardiac surgery. International Journal of Vascular Surgery and Medicine. 2017; 3(2):26-32.
- Cury JL, Brunetto AF, Aydos RD. Negative effects of chronic kidney failure on lung function and functional capacity. Brazilian Journal of Physical Therapy. 2010; 14(2):91-8. [DOI:10.1590/S1413-35552010005000008] [PMID]
- Mehrotra R, Chiu YW, Kalantar Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Archives of Internal Medicine. 2011; 171(2):110-8. [DOI:10.1001/archinternmed.2010.352] [PMID]
- LeMone P, Burke K, Dwyer T, Levett-Jones T, Moxham L, Reid Searl K, et al. Medical-surgical nursing: Critical thinking for person-centred care. London: Pearson; 2016.
- De Broe ME, Lins RL, De Backer WA. Pulmonary aspects of dialysis patients. Replacement of Renal Function by Dialysis. Berlin: Springer; 2004. [DOI:10.1007/978-1-4020-2275-3_34]
- Thenappan T. Pulmonary hypertension in chronic kidney disease: A hemodynamic characterization. Thousand Oaks, California: SAGE; 2017. [doi.org/10.1177/2045893217728462]
- Rahgoshai R, Rahgoshai R, Khosraviani A, Nasiri AA, Solouki M. Acute effects of hemodialysis on pulmonary function in patients with end-stage renal disease. Iranian Journal of Kidney Diseases. 2010; 4(3):214-7. [PMID]
- Pierson DJ. Respiratory considerations in the patient with renal failure. Respiratory Care. 2006; 51(4):413-22. [PMID]
- Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC: Cardiovascular Imaging. 2010; 3(6):586-94. [DOI:10.1016/j.jcmg.2010.02.005]
- Mousavi SA, Mahdavi Mazdeh M, Yahyazadeh H, Azadi M, Rahimzadeh N, Yoosefnejad H, et al. Pulmonary hypertension and predisposing factors in patients receiving hemodialysis. Iranian Journal of Kidney Diseases. 2008; 2(1):29-33. [PMID]
- Kovelis D, Pitta F, Probst VS, Peres CPA, Delfino VDA, Mocelin AJ, et al. Pulmonary function and respiratory muscle strength in chronic renal failure patients on hemodialysis. Jornal Brasileiro de Pneumologia. 2008; 34(11):907-12. [DOI:10.1590/S1806-37132008001100004] [PMID]
- Gosselink R, Schrever K, Cops P, Witvrouwen H, De Leyn P, Troosters T, et al. Incentive spirometry does not enhance recovery after thoracic surgery. Critical Care Medicine. 2000; 28(3):679-83. [DOI:10.1097/00003246-200003000-00013] [PMID]
- Basoglu OK, Atasever A, Bacakoglu F. The efficacy of incentive spirometry in patients with COPD. Respirology. 2005; 10(3):349-53. [DOI:10.1111/j.1440-1843.2005.00716.x] [PMID]
- Carvalho CR, Paisani DM, Lunardi AC. Incentive spirometry in major surgeries: A systematic review. Brazilian Journal of Physical Therapy. 2011; 15(5):343-50. [DOI:10.1590/S1413-35552011005000025] [PMID]
- Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Physical Therapy. 1994; 74(1):3-10. [DOI:10.1093/ptj/74.1.3]
- Westwood K, Griffin M, Roberts K, Williams M, Yoong K, Digger T. Incentive spirometry decreases respiratory complications following major abdominal surgery. The Surgeon. 2007; 5(6):339-42. [DOI:10.1016/S1479-666X(07)80086-2]
- Pierce R, Johns D. Spirometry: The measurement and interpretation of ventilatory function in clinical practice. Melbourne: National Asthma Campaign; 1995.
- Haeffener MP, Ferreira GM, Barreto SSM, Arena R, Dall’Ago P. Incentive spirometry with expiratory positive airway pressure reduces pulmonary complications, improves pulmonary function and 6-minute walk distance in patients undergoing coronary artery bypass graft surgery. American Heart Journal. 2008; 156(5):900.e1-900
- Kotani T, Akazawa T, Sakuma T, Nagaya S, Sonoda M, Tanaka Y, et al. Effects of incentive spirometry on respiratory motion in healthy subjects using cine breathing magnetic resonance imaging. Annals of Rehabilitation Medicine. 2015; 39(3):360-5. [DOI:10.5535/arm.2015.39.3.360] [PMID] [PMCID]
- Agostini P, Naidu B, Cieslik H, Steyn R, Rajesh PB, Bishay E, et al. Effectiveness of incentive spirometry in patients following thoracotomy and lung resection including those at high risk for developing pulmonary complications. Thorax. 2013; 68(6):580-5. [DOI:10.1136/thoraxjnl-2012-202785] [PMID]
- Navari K, Farshidi H, Pour Reza Gholi F. Spirometry parameters in patients undergoing hemodialysis with bicarbonate and acetate dialysates. Iranian Journal of Kidney Diseases. 2008; 2(3):149-53. [PMID] [DOI:10.1016/j.ahj.2008.08.006]
Article type: Original Research Articles |
Subject:
Nursing
Received: 2018/01/28 | Accepted: 2018/06/2 | Published: 2018/09/1
Received: 2018/01/28 | Accepted: 2018/06/2 | Published: 2018/09/1
References
1. Tamadon MR, Adibimehr AR, Ghorbani R. Cognitive impairment in dialysis and non-dialysis patients suffering from chronic renal failure and comparing them with a control group. Middle East Journal of Rehabilitation and Health Studies. 2017; 4(4):e12815. [DOI:10.5812/mejrh.12815] [DOI:10.5812/mejrh.12815]
2. Durgun B, Yüksel A, Erol G, Kobuk M, Doğancı S. Acute on chronic renal failure has worse postoperative outcomes than end-stage renal disease following cardiac surgery. International Journal of Vascular Surgery and Medicine. 2017; 3(2):26-32.
3. Cury JL, Brunetto AF, Aydos RD. Negative effects of chronic kidney failure on lung function and functional capacity. Brazilian Journal of Physical Therapy. 2010; 14(2):91-8. [DOI:10.1590/S1413-35552010005000008] [PMID] [DOI:10.1590/S1413-35552010005000008]
4. Mehrotra R, Chiu YW, Kalantar Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Archives of Internal Medicine. 2011; 171(2):110-8. [DOI:10.1001/archinternmed.2010.352] [PMID] [DOI:10.1001/archinternmed.2010.352]
5. LeMone P, Burke K, Dwyer T, Levett-Jones T, Moxham L, Reid Searl K, et al. Medical-surgical nursing: Critical thinking for person-centred care. London: Pearson; 2016.
6. De Broe ME, Lins RL, De Backer WA. Pulmonary aspects of dialysis patients. Replacement of Renal Function by Dialysis. Berlin: Springer; 2004. [DOI:10.1007/978-1-4020-2275-3_34] [DOI:10.1007/978-1-4020-2275-3_34]
7. Thenappan T. Pulmonary hypertension in chronic kidney disease: A hemodynamic characterization. Thousand Oaks, California: SAGE; 2017. [doi.org/10.1177/2045893217728462]
8. Rahgoshai R, Rahgoshai R, Khosraviani A, Nasiri AA, Solouki M. Acute effects of hemodialysis on pulmonary function in patients with end-stage renal disease. Iranian Journal of Kidney Diseases. 2010; 4(3):214-7. [PMID] [PMID]
9. Pierson DJ. Respiratory considerations in the patient with renal failure. Respiratory Care. 2006; 51(4):413-22. [PMID] [PMID]
10. Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, et al. Detection of pulmonary congestion by chest ultrasound in dialysis patients. JACC: Cardiovascular Imaging. 2010; 3(6):586-94. [DOI:10.1016/j.jcmg.2010.02.005] [DOI:10.1016/j.jcmg.2010.02.005]
11. Mousavi SA, Mahdavi Mazdeh M, Yahyazadeh H, Azadi M, Rahimzadeh N, Yoosefnejad H, et al. Pulmonary hypertension and predisposing factors in patients receiving hemodialysis. Iranian Journal of Kidney Diseases. 2008; 2(1):29-33. [PMID] [PMID]
12. Kovelis D, Pitta F, Probst VS, Peres CPA, Delfino VDA, Mocelin AJ, et al. Pulmonary function and respiratory muscle strength in chronic renal failure patients on hemodialysis. Jornal Brasileiro de Pneumologia. 2008; 34(11):907-12. [DOI:10.1590/S1806-37132008001100004] [PMID] [DOI:10.1590/S1806-37132008001100004]
13. Gosselink R, Schrever K, Cops P, Witvrouwen H, De Leyn P, Troosters T, et al. Incentive spirometry does not enhance recovery after thoracic surgery. Critical Care Medicine. 2000; 28(3):679-83. [DOI:10.1097/00003246-200003000-00013] [PMID] [DOI:10.1097/00003246-200003000-00013]
14. Basoglu OK, Atasever A, Bacakoglu F. The efficacy of incentive spirometry in patients with COPD. Respirology. 2005; 10(3):349-53. [DOI:10.1111/j.1440-1843.2005.00716.x] [PMID] [DOI:10.1111/j.1440-1843.2005.00716.x]
15. Carvalho CR, Paisani DM, Lunardi AC. Incentive spirometry in major surgeries: A systematic review. Brazilian Journal of Physical Therapy. 2011; 15(5):343-50. [DOI:10.1590/S1413-35552011005000025] [PMID] [DOI:10.1590/S1413-35552011005000025]
16. Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysis. Physical Therapy. 1994; 74(1):3-10. [DOI:10.1093/ptj/74.1.3] [DOI:10.1093/ptj/74.1.3]
17. Westwood K, Griffin M, Roberts K, Williams M, Yoong K, Digger T. Incentive spirometry decreases respiratory complications following major abdominal surgery. The Surgeon. 2007; 5(6):339-42. [DOI:10.1016/S1479-666X(07)80086-2] [DOI:10.1016/S1479-666X(07)80086-2]
18. Pierce R, Johns D. Spirometry: The measurement and interpretation of ventilatory function in clinical practice. Melbourne: National Asthma Campaign; 1995.
19. Haeffener MP, Ferreira GM, Barreto SSM, Arena R, Dall'Ago P. Incentive spirometry with expiratory positive airway pressure reduces pulmonary complications, improves pulmonary function and 6-minute walk distance in patients undergoing coronary artery bypass graft surgery. American Heart Journal. 2008; 156(5):900.e1-900 [DOI:10.1016/j.ahj.2008.08.006] [PMID]
20. Kotani T, Akazawa T, Sakuma T, Nagaya S, Sonoda M, Tanaka Y, et al. Effects of incentive spirometry on respiratory motion in healthy subjects using cine breathing magnetic resonance imaging. Annals of Rehabilitation Medicine. 2015; 39(3):360-5. [DOI:10.5535/arm.2015.39.3.360] [PMID] [PMCID] [DOI:10.5535/arm.2015.39.3.360]
21. Agostini P, Naidu B, Cieslik H, Steyn R, Rajesh PB, Bishay E, et al. Effectiveness of incentive spirometry in patients following thoracotomy and lung resection including those at high risk for developing pulmonary complications. Thorax. 2013; 68(6):580-5. [DOI:10.1136/thoraxjnl-2012-202785] [PMID] [DOI:10.1136/thoraxjnl-2012-202785]
22. Navari K, Farshidi H, Pour Reza Gholi F. Spirometry parameters in patients undergoing hemodialysis with bicarbonate and acetate dialysates. Iranian Journal of Kidney Diseases. 2008; 2(3):149-53. [PMID] [DOI:10.1016/j.ahj.2008.08.006] [DOI:10.1016/j.ahj.2008.08.006]
Send email to the article author