Volume 21, Issue 4 (December 2023)
Iranian Rehabilitation Journal 2023, 21(4): 591-600 |
Back to browse issues page
Ethics code: IR.USWR.REC.1400.095
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azarnia S, Ezatti K, Naghdi S, Abdollahi I, Shanbehzadeh S, Baharloueii H et al . The Effect of Concurrent Transcranial Direct Current Stimulation and Robotic Training of the Upper Limb in Stroke Recovery: A Systematic Review and Meta-analysis. Iranian Rehabilitation Journal 2023; 21 (4) :591-600
URL: http://irj.uswr.ac.ir/article-1-1765-en.html
URL: http://irj.uswr.ac.ir/article-1-1765-en.html
Somaye Azarnia * 

 1, Kamran Ezatti2
1, Kamran Ezatti2 

 , Soofia Naghdi3
, Soofia Naghdi3 

 , Iraj Abdollahi4
, Iraj Abdollahi4 

 , Sanaz Shanbehzadeh5
, Sanaz Shanbehzadeh5 

 , Hamzeh Baharloueii3
, Hamzeh Baharloueii3 

 , Shapoor Jaberzadeh6
, Shapoor Jaberzadeh6 




 1, Kamran Ezatti2
1, Kamran Ezatti2 

 , Soofia Naghdi3
, Soofia Naghdi3 

 , Iraj Abdollahi4
, Iraj Abdollahi4 

 , Sanaz Shanbehzadeh5
, Sanaz Shanbehzadeh5 

 , Hamzeh Baharloueii3
, Hamzeh Baharloueii3 

 , Shapoor Jaberzadeh6
, Shapoor Jaberzadeh6 


1- Student Research Committee, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
2- Department of Physiotherapy, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Musculoskeletal Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Physiotherapy, School of Rehabilitation, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
5- Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran.
6- Department of Physiotherapy, Faculty of Medicine, Monash University, Melbourne, Australia.
2- Department of Physiotherapy, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
3- Musculoskeletal Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Physiotherapy, School of Rehabilitation, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran.
5- Rehabilitation Research Center, Iran University of Medical Sciences, Tehran, Iran.
6- Department of Physiotherapy, Faculty of Medicine, Monash University, Melbourne, Australia.
Full-Text [PDF 1047 kb]
(207 Downloads)
| Abstract (HTML) (966 Views)
Full-Text: (66 Views)
Introduction
Stroke is one of the main health problems in the world and one of the most debilitating neurological diseases in adults [1]. Stroke survivors suffer from persistent motor, perceptual, and somatosensory complications. Long-lasting physical functional impairment and limitations in daily living activities drive the compelling advances in rehabilitation systems to improve patients’ motor functioning.
Stroke induces significant changes to the synaptic system which may lead to changes in cerebral plasticity. Stroke rehabilitation interventions use the central nervous system (CNS) neuroplasticity capacity to promote functional recovery. tDCS is considered to be a type of neuromodulatory and functions by using weak direct currents that pass through the skull and affect the cortex and deeper brain centers. Under certain current parameters, anodal stimulation increases cortical excitability (depolarization), and cathodal stimulation leads to decreased excitability (hyperpolarization) [2, 3]. The polarity-dependent effects of tDCS enable physiotherapists to apply the current in different ways to increase motor function in patients who have experienced stroke by considering the abnormal balance of motor cortex excitability after stroke, the affected hemisphere’s low excitability, and healthy hemisphere’s high excitability [4]. Strong evidence suggests that specific movement training can help restore motor function by adapting the neural plasticity using high-dose intensive task-specific training [3]. A study conducted by Silva et al. [5]. suggested that to amplify clinical effects and reach results that are more effective and long-lasting, a combination of therapeutic tools should be used. The development of robotic technology in recent years enables clinicians to provide training with high intensity and dosage. A robot is defined as a multi-purpose and re-programmable manipulator designed to move parts of the body through variable programmed movements to perform a task [6]. Many studies have used concurrent RT and tDCS to improve the performance of stroke patients; however, the results of the studies are inconsistent. Therefore, a systematic review of the literature is required to evaluate the effects of concurrent RT and tDCS on the upper limb function of patients who have experienced stroke. This review compares the effects of concurrent RT and tDCS with RT alone, on upper limb function in stroke patients.
Materials and Methods
Protocol and registration
Our study protocol has been registered in the PROSPERO database under the ID=CRD42020205148
Search strategy
We included clinical trials by searching electronic databases including Web of Science, CENTRAL, EMBASE, PubMed, Scopus, and Physiotherapy Evidence Database (PEDro) from 2000 to January 1, 2021. Search terms were established with the subject headings and keywords: (Stroke OR “cerebral vascular accident” OR “brain infarct* OR hemiplegia*) AND (“transcranial direct current stimulation” OR tDCS) AND “robotic training” OR “robotic-assisted therapy”. We used these terms in various combinations and also, hand searched the references that were used in the included study.
Study selection
The titles and abstracts that were retrieved through the search were screened by two reviewers. Two members of the review team assessed full texts retrieved from each study for eligibility separately. Discussion with a third reviewer was done if consensus was not reached during the discussion about disagreements over eligibility. We included studies that used tDCS (anodal. cathodal, dual) in combination with robotic-assisted therapy in stroke patients and published in English.
Eligibility criteria
Studies recruiting individuals with all types of stroke and recovery phases and all tDCS interventions (frequency, duration, intensity, or montage) were included. Studies that involved healthy participants, patients with aphasia or cognitive impairment, any other neurological diagnosis, and studies assessing the effect of tDCS in combination with other therapies rather than robotic training were excluded.
Data extraction
Two reviewers screened the titles and abstracts retrieved to remove irrelevant studies. Data including study authors, year of publication, study design, stroke type, mean time since stroke (days), tDCS intensity (mA), tDCS duration (min), tDCS montage, follow-up time, Mean±SD of the motor function outcomes, and several subjects in each group were extracted from the studies that were included in this review. If the data was reported as the standard error of the mean, it was transformed to SD. Data extraction was done by two independent authors directly from the full text of these studies. If no consensus was achieved over the disagreements arising in this area, a third author’s opinion was sought after.
Quality assessment
The quality of selected studies was evaluated by two authors (Somaye Azarnia and Sanaz Shanbezade) using the Cochrane collaboration tool for assessing risk-of-bias as outlined in the Cochrane handbook for systematic reviews of interventions [7]. The overall risk of bias for each study was evaluated as low if all key fields were assessed low; high when one or more key field was assessed high; and unclear when one or more key field was assessed unclear. Any disagreement between the authors on the methodological quality of the identified studies was resolved by discussing with a third reviewer (AT). We contacted the authors of the included studies for any additional information on the study methods.
Data analysis
All analyses were performed in Stata software, version 15. Mean±SD were used for meta-analysis. Evaluation of 95% CI and calculation of pooled effect sizes (ES) reported as standardized mean difference was done by using the random-effect model. Cohen’s d ES was used and interpreted as follows: Small (0.2–0.5), moderate (0.5–0.8), and large (>0.8). The I2 statistics were used to assess the presence of heterogeneity through the included studies and were interpreted as high I2≥75%, moderate I2≥50%, and low I2≤25%. Sensitivity analyses were performed if there was high heterogeneity to explore the source of heterogeneity. These analyses included a leave-one-out approach by omitting each study and subgroup analysis based on the stage of recovery and the outcome measure used for functional evaluation.
Results
Data overview
A total of 2678 articles were found in the initial search. After eliminating 792 duplicates, 1871 studies were screened. Eight additional studies were excluded after a review of the full texts (Figure 1). Based on the eligibility criteria, 15 articles were included. Four studies did not report the results of sham stimulation and were removed by reading the full text [8, 9]. Four studies were congress articles Pistarini [10], Hesse et al. [8], Edwards [2], Triccas et al. [11] and were removed. Finally, 7 studies were included in this study (Figure 1).
Method of quality assessment
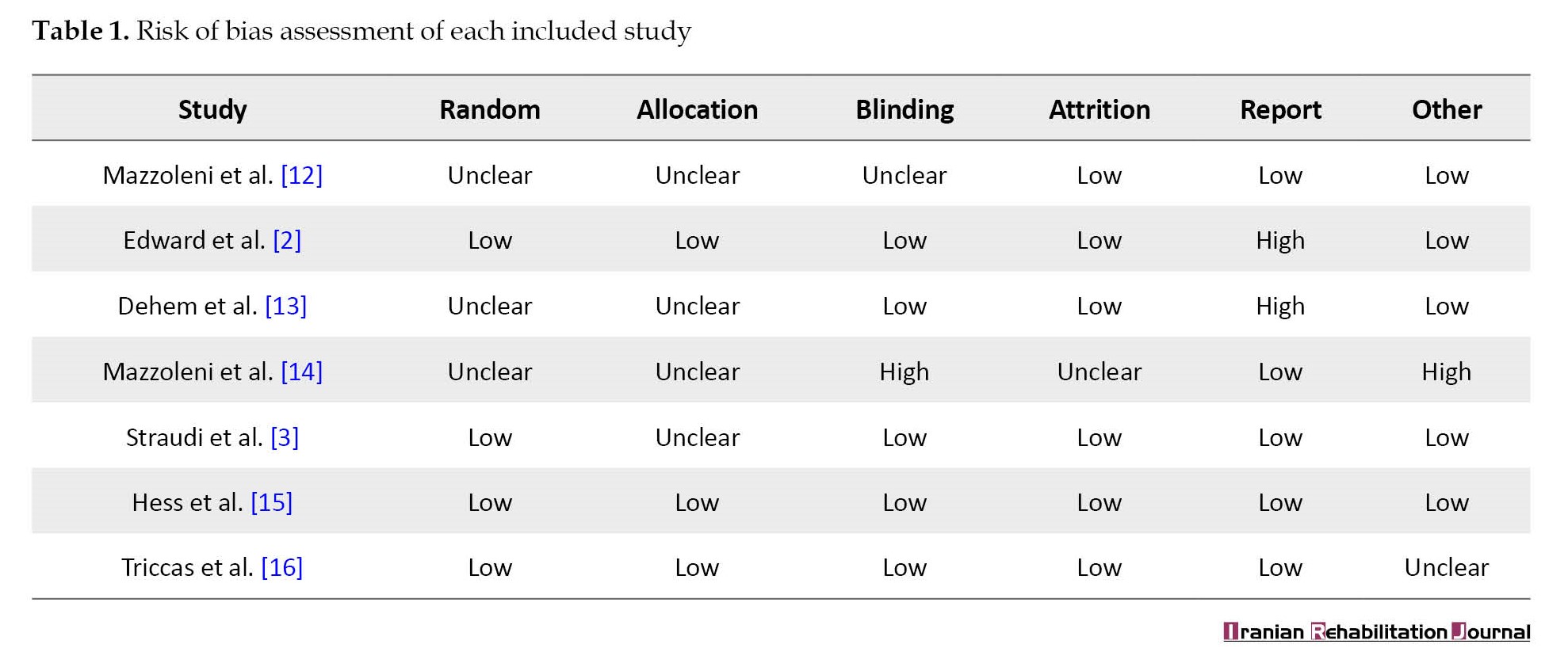
The Cochrane collaboration risk of bias tool was used by the authors to assess the risk of bias among the studies. This tool includes blinding of participants and personnel, allocation concealment, random sequence generation, blinding of outcome assessment, incomplete outcome data, and selective reporting. The quality assessment of seven studies is shown in Table 1.
We presented the risk of bias for each comparison separately in Figure 2.
Participants characteristics
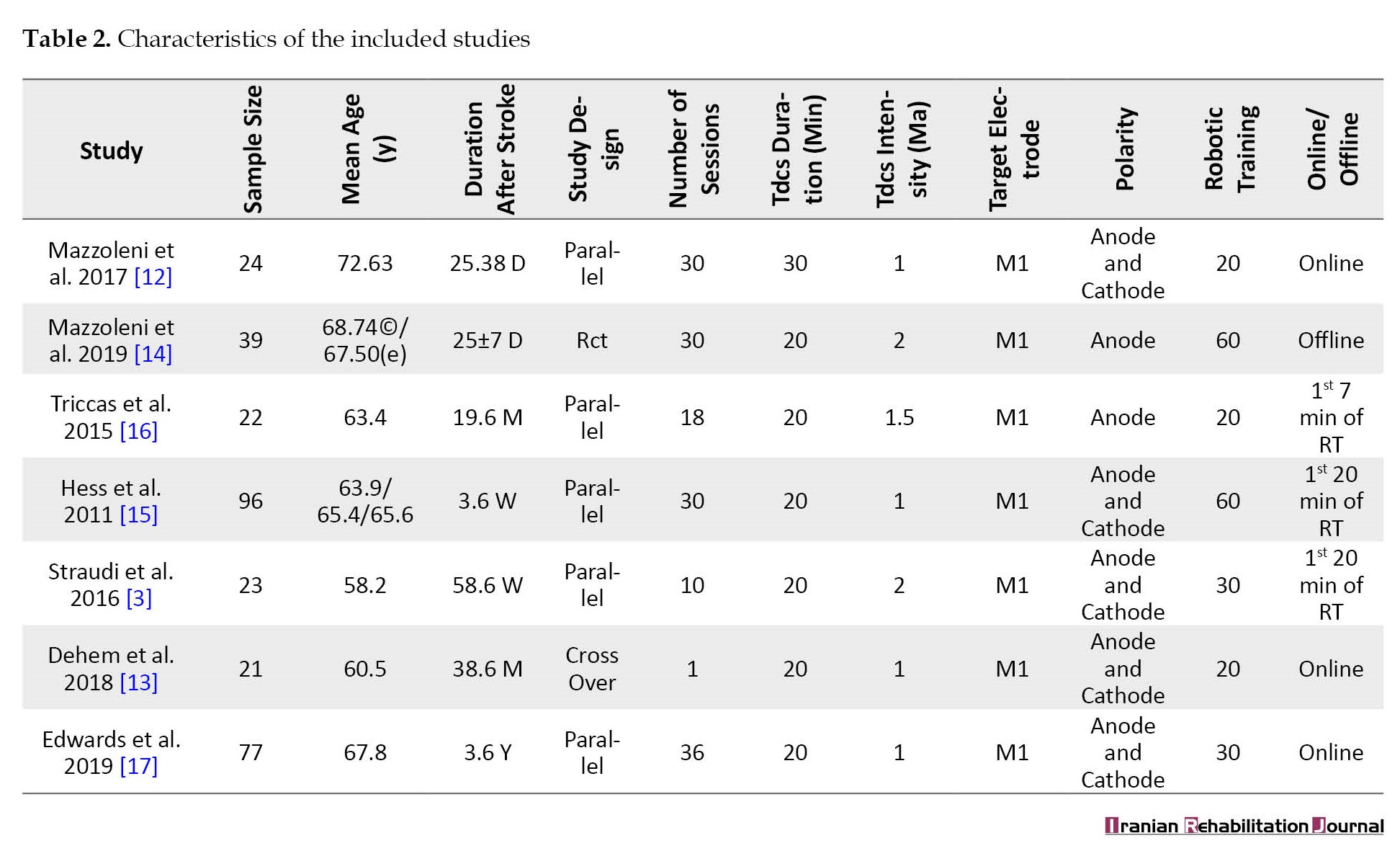
In total, there were 302 individuals in the studies of this review. The mean age was between 58.2 and 72.63 years and 172 participants were male. The characteristics of these studies are demonstrated in Table 2.
tDCS intervention
Two studies applied anodal tDCS [14, 15, 16], and the effect of dual tDCS and usage of anodal tDCS over the side that was affected and cathodal tDCS over the non-affected side were analyzed in five studies [3, 12, 13, 15, 17] (Table 2).
In terms of tDCS duration, two studies applied 30 min of tDCS [13, 3], and the other five studies used 20 min [12-14] (Table 2). The overall number of sessions was between 1 to 36 sessions. The primary motor cortex (M1) was the site of expiatory stimulation [2, 13, 3, 16]. In most studies, tDCS and RT were applied simultaneously (online effect) [13-18], while only one study applied tDCS before other interventions and assessment (offline effect) [11].
Assessment
Outcome measures
The main outcome measure was motor function [13, 16]. Several functional assessment tools were used in the included studies: Six studies used the Fugel-Meyer scale (FM) to assess motor functioning [2, 13, 3, 14], four studies reported the score of box and block test (B & B), which measures the gross manual dexterity of the patients, [13, 3, 16] and two studies used motor activity log to assess the quality of movement during activities of daily living [3, 14]. Three studies evaluated the effects of concurrent tDCS and RT on spasticity [12, 14, 15].
Results of meta-analysis
Functional performance: Overall effect of concurrent tDCS and RT
The pooled results on upper limb function showed that concurrent tDCS and RT had a moderate effect on motor function in comparison to RT alone in stroke patients, however, this effect was not significant (SMD=0.31, 95% CI, -0.20%, 0.83%, I2=84.1%) (Figure 3).
Moreover, no change was seen in the results related to the subgroup analysis based on the stage of recovery (acute/subacute or chronic). While subgroup analysis based on the instrument used (FM or B & B) reduced heterogeneity in the FM group (I2=0%). The results of sensitivity analysis revealed no change in the results of meta-analysis by excluding each study. The asymmetrical funnel plot suggests publication bias, however, Egger’s test was not statistically significant and suggested no publication bias for functional performance studies (coefficient=0.8782371; P=0.78).
Spasticity
Three studies examined the effect of concurrent tDCS and RT on spasticity. These studies failed to show any significant effect (SMD=0.12, 95% CI, -0.47%, 0.23%, I2=0%) (Figure 4) [13, 15, 16].
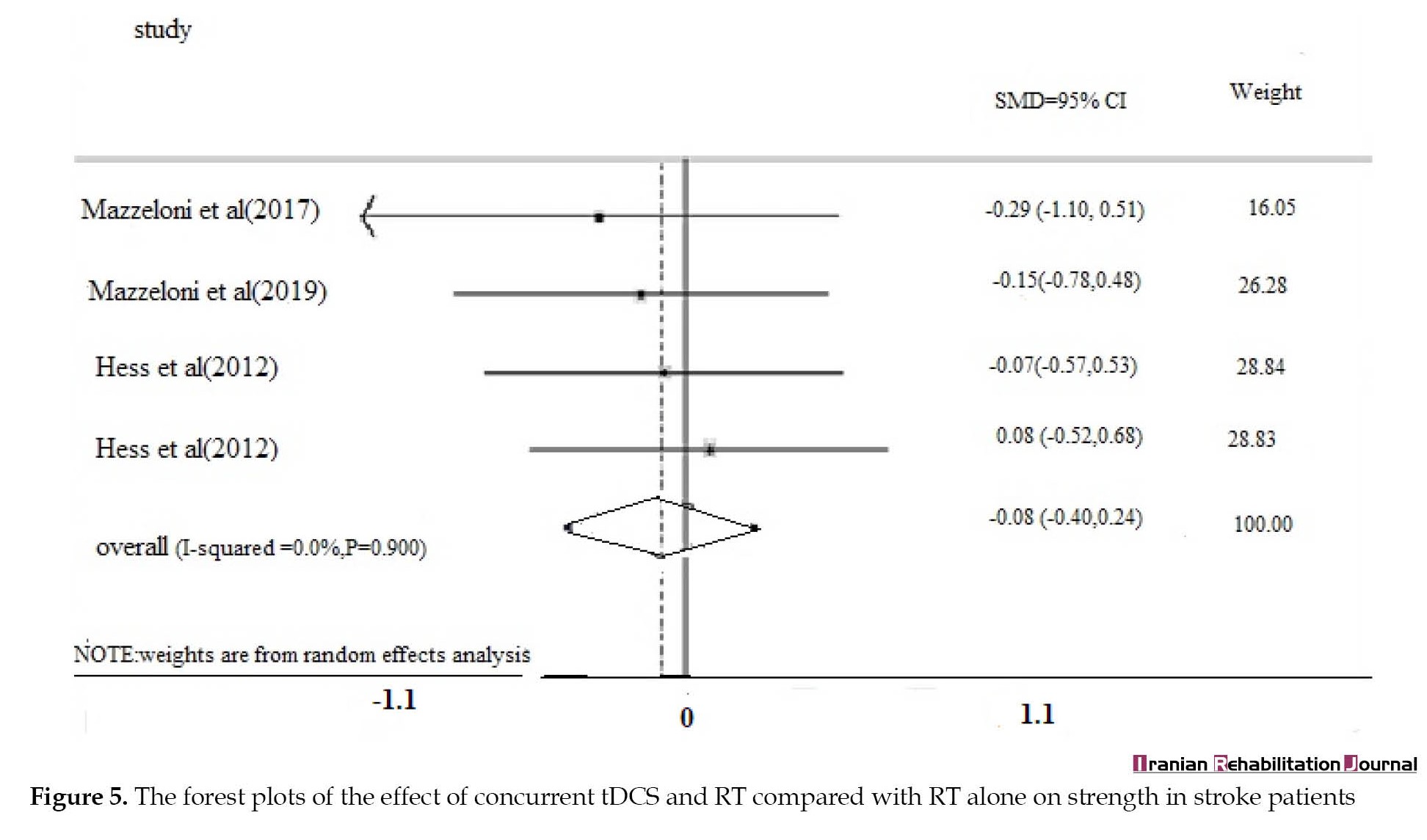
Muscle strength
Four studies examined the effect of concurrent tDCS and RT on muscle strength, the overall pooled results showed that there was no significant difference in concurrent therapy compared to RT (SMD=0.08, 95% CI, -0.40%, 0.24%, I2=0%) (Figure 5).
Discussion
Seven articles with 302 participants were included in this systematic review and meta-analysis which compares the effects of concurrent tDCS and RT with RT alone on the upper limb motor function of stroke patients. According to the study results, no difference was obtained in the efficacy of concurrent tDCS and RT compared to RT on upper limb/hand function in survivors of stroke. Additionally, no other effect was observed for reducing spasticity and increasing muscle strength with concurrent therapy compared to RT. Although a systematic review that was conducted previously on the effect of RT has shown improved upper limb motor function in patients with stroke [18], concurrent tDCS and RT had no additional effects. Bertani et al. showed that RT tends to show more effective results in improving the motor function of stroke patients compared to using conventional therapy to reach the same goal [18]. Changes in neural activation and functional connections might be related to the movement and therapy-mediated effect of RT [13]. In addition, a review study demonstrated the efficacy of tDCS on motor function of the upper extremities in patients with stroke. tDCS is a non-invasive stimulation of the weak electrical current brain, which can be used to manipulate membrane potential and modulate the spontaneous firing rate of neurons in humans [19]. tDCS may also reduce the hemispheric imbalance in stroke patients which enhances improved brain modulation [20].
The results of the present study could be explained by two reasons. The first reason is related to the neuroplastic effects of concurrent tDCS and RT. The metaplasticity, Hebbian plasticity, and ceiling effects must be taken into account when combining TDCS and RT as neurorehabilitation approaches [21]. The way synaptic plasticity can be modulated by prior synaptic [22] and the reversal of previously induced synaptic plasticity is described by a term called ‘metaplasticity’, which could limit the effect of an intervention. When applying two treatment strategies concurrently, hemostatic metaplasticity can reduce the effect of the latter strategy to maintain the neural network activity within a physiologic range. According to Hebbian’s hypothesis, as synaptic stimulation increases and neurotransmitters are released, postsynaptic neurons are changed. This leads to desensitization of neurons and saturation of receptors, not increasing excitatory postsynaptic potentials [23]. Therefore, this might also limit the effect of concurrent tDCS and RT. The second reason could be attributed to the confounding factors which have not been controlled in the studies and could impact the efficacy of an intervention including the location and size of the lesion in stroke patients, type of stroke, and time since stroke. Both the spontaneous and therapeutic-induced mechanisms of plasticity can promote post-stroke recovery. In the second epoch of recovery, spontaneous brain recovery is dominant; however, recovery in the third epoch (chronic phase of brain repair) is mostly related to brain plasticity, hence, it is important to limit the time since stroke.
Conclusion
Overall, the results indicated no additional effect of concurrent tDCS and RT compared to RT alone on the enhancement of upper limb function in stroke patients. The therapeutic protocols and methodology used in the included studies were highly heterogeneous and prevented conclusive conclusions.
Limitations
The findings in the current study should be interpreted considering the following limitations. Only articles published in English were included in this review. There may be other studies in this field that were not included in this study. The low number of studies in the field, and low sample size in the majority of these studies is another limitation that should be considered. Another limitation is the scarce number of articles in this field, which were mostly low quality, and evaluated motor function after a short duration of intervention, and most studies did not have a sample size justification for the primary outcome measures.
Suggestions for future studies
The study of concurrent tDCS and RT represents a young field of interest and the number of articles is limited. Therefore, we suggest that the effect of combining these two methods be investigated by type of stroke (ischemic, hemorrhagic), lesion of stroke (cortical or subcortical), and stage of stroke (acute, chronic). Future studies should focus more on the aspects that are mentioned previously.
Ethical Considerations
Compliance with ethical guidelines
The research protocol was approved by the Ethics Committee of University of Social Welfare and Rehabilitation Science (Code: IR.USWR.REC.1400.095).
Funding
This study was financially supported by the Research Committee of the University of Social Welfare and Rehabilitation Science (No.: 2620/ت/00).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Stroke is one of the main health problems in the world and one of the most debilitating neurological diseases in adults [1]. Stroke survivors suffer from persistent motor, perceptual, and somatosensory complications. Long-lasting physical functional impairment and limitations in daily living activities drive the compelling advances in rehabilitation systems to improve patients’ motor functioning.
Stroke induces significant changes to the synaptic system which may lead to changes in cerebral plasticity. Stroke rehabilitation interventions use the central nervous system (CNS) neuroplasticity capacity to promote functional recovery. tDCS is considered to be a type of neuromodulatory and functions by using weak direct currents that pass through the skull and affect the cortex and deeper brain centers. Under certain current parameters, anodal stimulation increases cortical excitability (depolarization), and cathodal stimulation leads to decreased excitability (hyperpolarization) [2, 3]. The polarity-dependent effects of tDCS enable physiotherapists to apply the current in different ways to increase motor function in patients who have experienced stroke by considering the abnormal balance of motor cortex excitability after stroke, the affected hemisphere’s low excitability, and healthy hemisphere’s high excitability [4]. Strong evidence suggests that specific movement training can help restore motor function by adapting the neural plasticity using high-dose intensive task-specific training [3]. A study conducted by Silva et al. [5]. suggested that to amplify clinical effects and reach results that are more effective and long-lasting, a combination of therapeutic tools should be used. The development of robotic technology in recent years enables clinicians to provide training with high intensity and dosage. A robot is defined as a multi-purpose and re-programmable manipulator designed to move parts of the body through variable programmed movements to perform a task [6]. Many studies have used concurrent RT and tDCS to improve the performance of stroke patients; however, the results of the studies are inconsistent. Therefore, a systematic review of the literature is required to evaluate the effects of concurrent RT and tDCS on the upper limb function of patients who have experienced stroke. This review compares the effects of concurrent RT and tDCS with RT alone, on upper limb function in stroke patients.
Materials and Methods
Protocol and registration
Our study protocol has been registered in the PROSPERO database under the ID=CRD42020205148
Search strategy
We included clinical trials by searching electronic databases including Web of Science, CENTRAL, EMBASE, PubMed, Scopus, and Physiotherapy Evidence Database (PEDro) from 2000 to January 1, 2021. Search terms were established with the subject headings and keywords: (Stroke OR “cerebral vascular accident” OR “brain infarct* OR hemiplegia*) AND (“transcranial direct current stimulation” OR tDCS) AND “robotic training” OR “robotic-assisted therapy”. We used these terms in various combinations and also, hand searched the references that were used in the included study.
Study selection
The titles and abstracts that were retrieved through the search were screened by two reviewers. Two members of the review team assessed full texts retrieved from each study for eligibility separately. Discussion with a third reviewer was done if consensus was not reached during the discussion about disagreements over eligibility. We included studies that used tDCS (anodal. cathodal, dual) in combination with robotic-assisted therapy in stroke patients and published in English.
Eligibility criteria
Studies recruiting individuals with all types of stroke and recovery phases and all tDCS interventions (frequency, duration, intensity, or montage) were included. Studies that involved healthy participants, patients with aphasia or cognitive impairment, any other neurological diagnosis, and studies assessing the effect of tDCS in combination with other therapies rather than robotic training were excluded.
Data extraction
Two reviewers screened the titles and abstracts retrieved to remove irrelevant studies. Data including study authors, year of publication, study design, stroke type, mean time since stroke (days), tDCS intensity (mA), tDCS duration (min), tDCS montage, follow-up time, Mean±SD of the motor function outcomes, and several subjects in each group were extracted from the studies that were included in this review. If the data was reported as the standard error of the mean, it was transformed to SD. Data extraction was done by two independent authors directly from the full text of these studies. If no consensus was achieved over the disagreements arising in this area, a third author’s opinion was sought after.
Quality assessment
The quality of selected studies was evaluated by two authors (Somaye Azarnia and Sanaz Shanbezade) using the Cochrane collaboration tool for assessing risk-of-bias as outlined in the Cochrane handbook for systematic reviews of interventions [7]. The overall risk of bias for each study was evaluated as low if all key fields were assessed low; high when one or more key field was assessed high; and unclear when one or more key field was assessed unclear. Any disagreement between the authors on the methodological quality of the identified studies was resolved by discussing with a third reviewer (AT). We contacted the authors of the included studies for any additional information on the study methods.
Data analysis
All analyses were performed in Stata software, version 15. Mean±SD were used for meta-analysis. Evaluation of 95% CI and calculation of pooled effect sizes (ES) reported as standardized mean difference was done by using the random-effect model. Cohen’s d ES was used and interpreted as follows: Small (0.2–0.5), moderate (0.5–0.8), and large (>0.8). The I2 statistics were used to assess the presence of heterogeneity through the included studies and were interpreted as high I2≥75%, moderate I2≥50%, and low I2≤25%. Sensitivity analyses were performed if there was high heterogeneity to explore the source of heterogeneity. These analyses included a leave-one-out approach by omitting each study and subgroup analysis based on the stage of recovery and the outcome measure used for functional evaluation.
Results
Data overview
A total of 2678 articles were found in the initial search. After eliminating 792 duplicates, 1871 studies were screened. Eight additional studies were excluded after a review of the full texts (Figure 1). Based on the eligibility criteria, 15 articles were included. Four studies did not report the results of sham stimulation and were removed by reading the full text [8, 9]. Four studies were congress articles Pistarini [10], Hesse et al. [8], Edwards [2], Triccas et al. [11] and were removed. Finally, 7 studies were included in this study (Figure 1).
Method of quality assessment
The Cochrane collaboration risk of bias tool was used by the authors to assess the risk of bias among the studies. This tool includes blinding of participants and personnel, allocation concealment, random sequence generation, blinding of outcome assessment, incomplete outcome data, and selective reporting. The quality assessment of seven studies is shown in Table 1.

We presented the risk of bias for each comparison separately in Figure 2.
Participants characteristics
In total, there were 302 individuals in the studies of this review. The mean age was between 58.2 and 72.63 years and 172 participants were male. The characteristics of these studies are demonstrated in Table 2.

tDCS intervention
Two studies applied anodal tDCS [14, 15, 16], and the effect of dual tDCS and usage of anodal tDCS over the side that was affected and cathodal tDCS over the non-affected side were analyzed in five studies [3, 12, 13, 15, 17] (Table 2).
In terms of tDCS duration, two studies applied 30 min of tDCS [13, 3], and the other five studies used 20 min [12-14] (Table 2). The overall number of sessions was between 1 to 36 sessions. The primary motor cortex (M1) was the site of expiatory stimulation [2, 13, 3, 16]. In most studies, tDCS and RT were applied simultaneously (online effect) [13-18], while only one study applied tDCS before other interventions and assessment (offline effect) [11].
Assessment
Outcome measures
The main outcome measure was motor function [13, 16]. Several functional assessment tools were used in the included studies: Six studies used the Fugel-Meyer scale (FM) to assess motor functioning [2, 13, 3, 14], four studies reported the score of box and block test (B & B), which measures the gross manual dexterity of the patients, [13, 3, 16] and two studies used motor activity log to assess the quality of movement during activities of daily living [3, 14]. Three studies evaluated the effects of concurrent tDCS and RT on spasticity [12, 14, 15].
Results of meta-analysis
Functional performance: Overall effect of concurrent tDCS and RT
The pooled results on upper limb function showed that concurrent tDCS and RT had a moderate effect on motor function in comparison to RT alone in stroke patients, however, this effect was not significant (SMD=0.31, 95% CI, -0.20%, 0.83%, I2=84.1%) (Figure 3).
Moreover, no change was seen in the results related to the subgroup analysis based on the stage of recovery (acute/subacute or chronic). While subgroup analysis based on the instrument used (FM or B & B) reduced heterogeneity in the FM group (I2=0%). The results of sensitivity analysis revealed no change in the results of meta-analysis by excluding each study. The asymmetrical funnel plot suggests publication bias, however, Egger’s test was not statistically significant and suggested no publication bias for functional performance studies (coefficient=0.8782371; P=0.78).
Spasticity
Three studies examined the effect of concurrent tDCS and RT on spasticity. These studies failed to show any significant effect (SMD=0.12, 95% CI, -0.47%, 0.23%, I2=0%) (Figure 4) [13, 15, 16].
Muscle strength
Four studies examined the effect of concurrent tDCS and RT on muscle strength, the overall pooled results showed that there was no significant difference in concurrent therapy compared to RT (SMD=0.08, 95% CI, -0.40%, 0.24%, I2=0%) (Figure 5).
Discussion
Seven articles with 302 participants were included in this systematic review and meta-analysis which compares the effects of concurrent tDCS and RT with RT alone on the upper limb motor function of stroke patients. According to the study results, no difference was obtained in the efficacy of concurrent tDCS and RT compared to RT on upper limb/hand function in survivors of stroke. Additionally, no other effect was observed for reducing spasticity and increasing muscle strength with concurrent therapy compared to RT. Although a systematic review that was conducted previously on the effect of RT has shown improved upper limb motor function in patients with stroke [18], concurrent tDCS and RT had no additional effects. Bertani et al. showed that RT tends to show more effective results in improving the motor function of stroke patients compared to using conventional therapy to reach the same goal [18]. Changes in neural activation and functional connections might be related to the movement and therapy-mediated effect of RT [13]. In addition, a review study demonstrated the efficacy of tDCS on motor function of the upper extremities in patients with stroke. tDCS is a non-invasive stimulation of the weak electrical current brain, which can be used to manipulate membrane potential and modulate the spontaneous firing rate of neurons in humans [19]. tDCS may also reduce the hemispheric imbalance in stroke patients which enhances improved brain modulation [20].
The results of the present study could be explained by two reasons. The first reason is related to the neuroplastic effects of concurrent tDCS and RT. The metaplasticity, Hebbian plasticity, and ceiling effects must be taken into account when combining TDCS and RT as neurorehabilitation approaches [21]. The way synaptic plasticity can be modulated by prior synaptic [22] and the reversal of previously induced synaptic plasticity is described by a term called ‘metaplasticity’, which could limit the effect of an intervention. When applying two treatment strategies concurrently, hemostatic metaplasticity can reduce the effect of the latter strategy to maintain the neural network activity within a physiologic range. According to Hebbian’s hypothesis, as synaptic stimulation increases and neurotransmitters are released, postsynaptic neurons are changed. This leads to desensitization of neurons and saturation of receptors, not increasing excitatory postsynaptic potentials [23]. Therefore, this might also limit the effect of concurrent tDCS and RT. The second reason could be attributed to the confounding factors which have not been controlled in the studies and could impact the efficacy of an intervention including the location and size of the lesion in stroke patients, type of stroke, and time since stroke. Both the spontaneous and therapeutic-induced mechanisms of plasticity can promote post-stroke recovery. In the second epoch of recovery, spontaneous brain recovery is dominant; however, recovery in the third epoch (chronic phase of brain repair) is mostly related to brain plasticity, hence, it is important to limit the time since stroke.
Conclusion
Overall, the results indicated no additional effect of concurrent tDCS and RT compared to RT alone on the enhancement of upper limb function in stroke patients. The therapeutic protocols and methodology used in the included studies were highly heterogeneous and prevented conclusive conclusions.
Limitations
The findings in the current study should be interpreted considering the following limitations. Only articles published in English were included in this review. There may be other studies in this field that were not included in this study. The low number of studies in the field, and low sample size in the majority of these studies is another limitation that should be considered. Another limitation is the scarce number of articles in this field, which were mostly low quality, and evaluated motor function after a short duration of intervention, and most studies did not have a sample size justification for the primary outcome measures.
Suggestions for future studies
The study of concurrent tDCS and RT represents a young field of interest and the number of articles is limited. Therefore, we suggest that the effect of combining these two methods be investigated by type of stroke (ischemic, hemorrhagic), lesion of stroke (cortical or subcortical), and stage of stroke (acute, chronic). Future studies should focus more on the aspects that are mentioned previously.
Ethical Considerations
Compliance with ethical guidelines
The research protocol was approved by the Ethics Committee of University of Social Welfare and Rehabilitation Science (Code: IR.USWR.REC.1400.095).
Funding
This study was financially supported by the Research Committee of the University of Social Welfare and Rehabilitation Science (No.: 2620/ت/00).
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
- Kuklina EV, Tong X, George MG, Bansil P. Epidemiology and prevention of stroke: A worldwide perspective. Expert Review of Neurotherapeutics. 2012; 12(2):199-208. [DOI:10.1586/ern.11.99] [PMID]
- Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restorative Neurology and Neuroscience. 2009; 27(3):199-207. [DOI:10.3233/RNN-2009-0470] [PMID] [PMCID]
- Straudi S, Fregni F, Martinuzzi C, Pavarelli C, Salvioli S, Basaglia N. tDCS and robotics on upper limb stroke rehabilitation: Effect modification by stroke duration and type of stroke. BioMed Research International. 2016; 2016:5068127. [DOI:10.1155/2016/5068127] [PMID] [PMCID]
- Kubis N. Non-invasive brain stimulation to enhance post-stroke recovery. Frontiers in Neural Circuits. 2016; 10:56. [DOI:10.3389/fncir.2016.00056] [PMID] [PMCID]
- da Silva TD, Fontes AMGG, de Oliveira-Furlan BS, Roque TT, Lima AII, de Souza BMM, et al. Effect of combined therapy of virtual reality and transcranial direct current stimulation in children and adolescents with cerebral palsy: A study protocol for a triple-blinded randomized controlled crossover trial. Frontiers in Neurology. 2020; 11:953. [PMID]
- Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008; 131(Pt 2):425-37. [DOI:10.1093/brain/awm311] [PMID]
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology. 2012; 41(3):818-27. [PMID]
- Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: A pilot study. Restorative Neurology and Neuroscience. 2007; 25(1):9-15. [PMID]
- Mazzoleni S, Do Tran V, Iardella L, Falchi E, Dario P, Posteraro F. Transcranial direct current stimulation and wrist robot-assisted integrated treatment on subacute stroke patients: A randomized, sham-controlled trial. Biosystems and Biorobotics. 2019; 21:518-22. [DOI:10.1007/978-3-030-01845-0_104]
- Pistarini C. Robot therapy and tDCS for recovery of movement in stroke patients. Paper presented at: Abu Dhabi Neuro Rehabilitation Conference. Meeting. June 29, 2015, Abu Dhabi, United Arab Emirates.
- Tedesco Triccas L. The effect of combining transcranial direct current stimulation with robot therapy for the impaired upper limb in stroke[ PhD dissertation]. Southampton: University of Southampton; 2014. [Link]
- Mazzoleni S, Tran VD, Iardella L, Dario P, Posteraro F. Randomized, sham-controlled trial based on transcranial direct current stimulation and wrist robot-assisted integrated treatment on subacute stroke patients: Intermediate results. IEEE. International Conference on Rehabilitation Robotics. 2017; 2017:555-560. [DOI:10.1109/ICORR.2017.8009306] [PMID]
- Dehem S, Gilliaux M, Lejeune T, Delaunois E, Mbonda P, Vandermeeren Y, et al. Effectiveness of a single session of dual-transcranial direct current stimulation in combination with upper limb robotic-assisted rehabilitation in chronic stroke patients: A randomized, double-blind, cross-over study. International Journal of Rehabilitation Research. 2018; 41(2):138-145. [DOI:10.1097/MRR.0000000000000274] [PMID]
- Mazzoleni S, Tran VD, Dario P, Posteraro F. Effects of transcranial direct current stimulation (tDCS) combined with wrist robot-assisted rehabilitation on motor recovery in subacute stroke patients: A randomized controlled trial. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2019; 27(7):1458-66. [DOI:10.1109/TNSRE.2019.2920576] [PMID]
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: An exploratory, randomized multicenter trial. Neurorehabilitation and Neural Repair. 2011; 25(9):838-46. [DOI:10.1177/1545968311413906] [PMID]
- Triccas LT, Burridge JH, Hughes A, Verheyden G, Desikan M, Rothwell J. A double-blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni-lateral robot therapy for the impaired upper limb in sub-acute and chronic stroke. NeuroRehabilitation. 2015; 37(2):181-91. [DOI:10.3233/NRE-151251] [PMID]
- Edwards DJ, Cortes M, Rykman-Peltz A, Chang J, Elder J, Thickbroom G, et al. Clinical improvement with intensive robot-assisted arm training in chronic stroke is unchanged by supplementary tDCS. Restorative Neurology and Neuroscience. 2019; 37(2):167-80. [DOI:10.3233/RNN-180869] [PMID]
- Bertani R, Melegari C, De Cola MC, Bramanti A, Bramanti P, Calabrò RS. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurological Sciences. 2017; 38(9):1561-9 [DOI:10.1007/s10072-017-2995-5] [PMID]
- Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restorative Neurology and Neuroscience. 2011; 29(6):463-92. [DOI:10.3233/RNN-2011-0618] [PMID]
- Kuo IJ, Tang CW, Tsai YA, Tang SC, Lin CJ, Hsu SP, et al. Neurophysiological signatures of hand motor response to dual-transcranial direct current stimulation in subacute stroke: A TMS and MEG study. Journal of Neuroengineering and Rehabilitation. 2020; 17(1):72. [DOI:10.1186/s12984-020-00706-1] [PMID]
- Takeuchi N, Izumi S. Combinations of stroke neurorehabilitation to facilitate motor recovery: Perspectives on Hebbian plasticity and homeostatic metaplasticity. Frontiers in Human Neuroscience. 2015; 9:349. [DOI:10.3389/fnhum.2015.00349] [PMID]
- Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nature Reviews. Neuroscience. 2008; 9(5):387. [DOI:10.1038/nrn2356] [PMID]
- Brown TH, Chattarji S. Hebbian synaptic plasticity: Evolution of the contemporary concept. In: Domany E, Hemmen JL, Schulten K, editors. Models of neural networks. Berlin: Springer; 1994. [DOI:10.1007/978-1-4612-4320-5_8]
Article type: Reviews |
Subject:
Physiotherapy
Received: 2022/09/4 | Accepted: 2022/12/25 | Published: 2023/12/1
Received: 2022/09/4 | Accepted: 2022/12/25 | Published: 2023/12/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |